Recently, new agents for the treatment of stage IV melanoma started coming in fast and furious. Despite these significant advances in therapy for melanoma, some oncologists are stuck in the past with their choice of therapies, which can be toxic, as well as have a dismal response rate. Add this to the already frightening confusion that sets in when a patient is diagnosed with melanoma, and the value of impartial but scientifically validated guidance is truly appreciated. Therefore, it is not surprising that patients and caregivers are often overwhelmed with the task of decision-making regarding their treatments. However, they can find reassurance and support when they turn to the Melanoma International Foundation for personalized navigation.
Immunotherapy
YERVOY. In March 2011, the FDA approved the first new immunotherapy for melanoma—Yervoy (generic name: ipilimumab)—which is administered intravenously by infusion to patients with advanced melanoma or with unresectable melanoma (that can’t be removed by surgery). In clinical trials, 15% to 20% of patients responded to treatment with Yervoy. Before the FDA approval of this immunotherapy—which uses the patient’s own immune system to fight the tumor—only 6% or less of patients responded to other types of therapies for melanoma. This was clearly a significant improvement in the number of patients who were responding positively to melanoma therapy. The side effects associated with Yervoy, and the slow pace of responding to the treatment, were at first notable hardships, with colitis (inflammation of the colon) being the most serious side effect. However, some patients have had long, positive responses to Yervoy and had no quality-of-life–changing side effects.
KEYTRUDA. Recently, the FDA approved 2 new immunotherapies for patients with melanoma, both are known as PD-1 inhibitors that block the body’s immune system from attacking melanoma tumors. The first PD-1 inhibitor is called Keytruda (pembrolizumab); it was approved in September 2014 and has shown a good response rate, with as many as 38% of patients responding positively to therapy with this drug.
OPDIVO. The second new PD-1 inhibitor, which was approved by the FDA in December 2014, is Opdivo (nivolumab), with as many as 32% of patients with melanoma responding positively to this therapy. The beauty of these immunotherapies is that their side effect profiles for the majority of patients with melanoma are minimally toxic, and that the tumor reduction with these drugs is quite promising. The drawback to these drugs is that they can be used as “third-line therapy,” meaning that before a patient with melanoma can receive one of these drugs, the patient must first try Yervoy or other treatments, and have disease progression despite receiving those treatments. In patients with a BRAF mutation (genetic alteration that prevents the patient from responding to some drugs), they must first use Yervoy and a BRAF inhibitor drug before being able to use either Keytruda or Opdivo. However, this sequence of drug therapies is expected to change once more robust data for these new immunotherapies are submitted to the FDA.
Braf Mutations
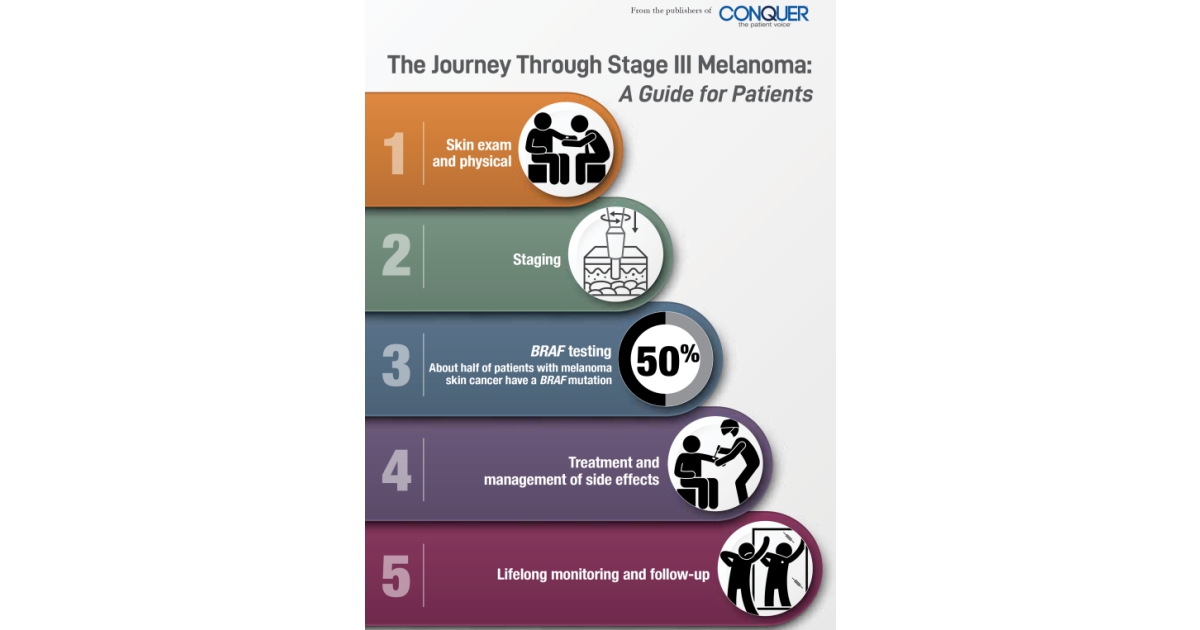
The advent of oral drugs that target the BRAF gene mutation of melanoma tumors arrived at the same time as the immunotherapy drugs. Approximately 50% of patients with melanoma have the BRAF mutation, which is related to intense sun exposure.
ZELBORAF. The first drug that led to the remission of melanoma for at least 6 months was the oral BRAF inhibitor Zelboraf (vemurafenib). The average survival of patients with advanced melanoma is approximately 9 months, but this length of survival was increased to 15.9 months with Zelboraf for many patients.
MEKINIST AND TAFLINLAR. The most notable BRAF-blocking combination therapy that had longer durable responses than Zelboraf is the combination of Mekinist (trametinib) and Taflinlar (dabrafenib). This BRAF/MEK inhibitor combination was approved by the FDA in January 2014 for use as first-line therapy for advanced or unresectable melanoma.
New MEK inhibitors are expected to be approved in the not-too-far future. Results from clinical trials have reports of patients with melanoma who had disease-free survival for more than 2 years with the new MEK inhibitors; however, these new drugs have sometimes caused fever, rash, sensitivity to sunlight, and muscle pain, among other adverse effects.
Just Around the Corner
The true revolution will come when the new therapies can be used in combination or in sequence to provide long-lasting survival without disease recurrence (disease coming back). There is also much work being done to figure out what patients have disease that is resistant to some therapies, and why.
The melanoma community is very pleased to have the attention of the research community at long last. With the continued excitement in melanoma breakthroughs, I’m optimistic that this discovery is just around the corner.
Patient Resources
- Melanoma International Foundation
http://melanomainternational.org/ - Patients Speak Out
http://melanomainternational.org/events-webinar/patient-experience-video - Patient/Caregiver Moderated Forum
www.melanomaforum.org - Immunotherapy
www.immunooncology.bmsinformation.com/how-io-is-different - National Cancer Institute
www.cancer.gov/cancertopics/types/melanoma - Clinical Trials
www.clinicaltrials.gov