May 2018 – Cancer Biomarkers and Molecular Testing

By Dana Taylor
Read about important advancements in tumor biomarkers and their role in guiding treatment in some types of cancer. Read More ›
By Kelsey Moroz
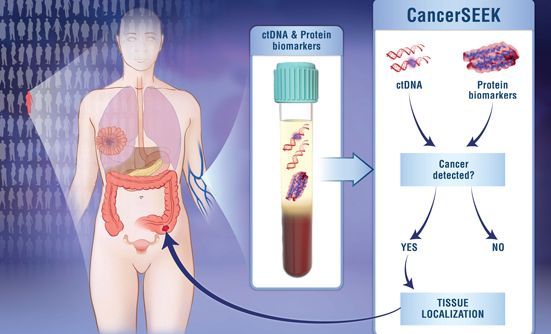
CancerSEEK is a noninvasive blood test that may help detect cancer in early stages, when treatment has the highest chance for success. Read More ›
Liquid biopsy is a noninvasive blood test that can help identify cancerous cells or biomarkers, such as gene mutations that may respond to targeted therapies. Read More ›
Targeted therapies for lung cancer are newer therapies designed to target specific types of genetic mutations found in some patients, and improve the response to treatment. Read More ›
In recent years, new treatments for acute myeloid leukemia have improved outcomes. In 2017, 2 new drugs were approved for patients with this type of blood cancer linked to a specific biomarker. Read More ›
The understanding of the role of dMMR or MSI-H biomarkers in cancer has led to the first approval of a cancer drug based on a biomarker instead of a specific tumor type.
Read More ›
Biomarkers are biologic markers that indicate changes in our genes and may increase the risk of cancer. Read More ›
Wenora Y. Johnson, 3-time survivor, shares her cancer story and encourages patients to ask their care team about biomarker testing. Read More ›
By Dana Taylor
This first-ever direct-to-consumer genetic test can provide information on potential risks to individuals who may not have access to genetic screening, but the information may be misleading without feedback from experts. Read More ›