Skin cancer continues to be the most common cancer diagnosed in men and women in the United States. By now, most Americans know about the importance of using appropriate precautions in the sun. The use of a strong sunblock and avoiding long periods of sun exposure are keys to preventing skin cancer, which can affect anyone, regardless of family history of skin cancer, race, or age. But knowing that we have to do something does not always translate to actually doing it.
According to the American Academy of Dermatology, about 9,500 Americans are diagnosed with skin cancer every day, and about 3 million Americans currently have skin cancer (not including melanoma, which can affect other parts of the body, not just the skin).
People who spend long periods in the sun, and those who have many skin moles, may need to have an annual checkup by a dermatologist to check for skin changes that may indicate early signs of skin cancer.
Merkel-Cell Carcinoma
One uncommon type of skin cancer that is, therefore, not well-known is called Merkel-cell carcinoma; although considered rare, about 1,600 people in the United States are diagnosed with this type of skin cancer every year, according to the National Cancer Institute.
As in all types of cancer, Merkel-cell carcinoma begins in the cells, specifically in cells called “Merkel cells”; it begins when those cells grow and multiply abnormally fast and out of control and become malignant.
According to the National Cancer Institute, Merkel cells are found on the top layer of the skin, close to the nerve endings of the skin, and are part of the neuroendocrine system. Although Merkel-cell carcinoma is less common than many other types of skin cancer, it tends to grow faster and is therefore more likely than other skin cancer types to spread, or metastasize, to other parts of the body early on. Overall, about half of patients will have disease recurrence (return), and about one-third of patients will have metastatic cancer.
Merkel-cell carcinoma usually initially spreads to the lymph nodes that are close to the skin area where the cancer begins, and then spreads to farther areas of the skin or body parts, including the lungs, brain, bones, and other body parts.
Sun Exposure and Weak Immune System
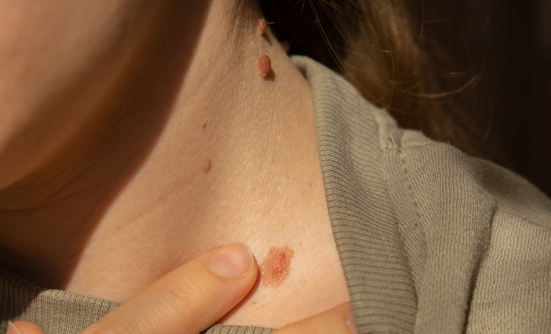
Like any other type of cancer, Merkel-cell carcinoma is more difficult to treat when it has spread to other parts of the body, which is why early diagnosis is so important. This type of skin cancer usually appears as a red, raised mole that is painless, firm, and dome-shaped on sun-exposed skin. Exposure to a lot of sun is the main risk factor.
In addition, people with a weak immune system—such as patients with cancer or with HIV infection, or people taking drugs that weaken the immune system—are at risk for Merkel-cell carcinoma.
Immunotherapy Brings New Hope
Until recently, the only treatment available for people with this type of skin cancer was chemotherapy, which did not provide much help for patients with advanced stages of Merkel-cell carcinoma.
In March 2017, the FDA approved the first drug—Bavencio (avelumab)—ever to receive approval specifically for the treatment of patients with metastatic Merkel-cell carcinoma. The FDA approved the use of Bavencio in patients aged 12 years or older who have metastatic Merkel-cell carcinoma, including those who have not received any chemotherapy.
Bavencio is a type of immunotherapy called “checkpoint inhibitors” that are targeting, or blocking, the PD-1/PD-L1 proteins that are found on immune cells, including cancer cells, which promote cancer growth. Blocking these proteins helps to stop the growth of cancer cells, including Merkel-cell carcinoma.
Commenting on the approval in 2017, Richard Pazdur, MD, Director of the FDA’s Oncology Center of Excellence, said, “While skin cancer is one of the most common cancers, patients with a rare form called Merkel cell carcinoma have not had an approved treatment option until now. The scientific community continues to make advances targeting the body’s immune system mechanisms for the treatment of various types of cancer. These advancements are leading to new therapies—even in rare forms of cancer where treatment options are limited or nonexistent.”
More recently, at the June 2018 meeting of the American Society of Clinical Oncology, researchers presented long-term results from 2 clinical trials using a checkpoint inhibitor, which led to extended life for half of the patients using these immunotherapies. One trial included the PD-L1 inhibitor Keytruda (pembrolizumab) and the second trial included Bavencio.
In both studies, about half of the patients with advanced or metastatic Merkel-cell carcinoma had better results and lived longer with the immunotherapy than with chemotherapy; starting treatment with immunotherapy immediately led to better results than starting with chemotherapy in those patients.
“This is so different from chemotherapy, and for the people who benefit, it’s an incredibly big change,” said Paul Nghiem, MD, of the University of Washington and Fred Hutchinson Cancer Research Center, Seattle, who presented the results at the meeting.
“These findings show that what we were hoping was real two years ago has indeed come true—for the people who initially responded to immunotherapy, it persisted to a far greater extent than for chemotherapy,” he added.
Nevertheless, Merkel-cell carcinoma does not respond to immunotherapy in every patient, Dr. Nghiem acknowledged, and more research is needed to find treatment for about half of the patients with this type of skin cancer whose disease doesn’t respond to checkpoint inhibitors, he said.
“Half of the people don’t persistently respond—that’s a huge number still. We’re trying hard to address that need,” Dr. Nghiem added, discussing ongoing research that he and his colleagues are conducting.
Key Points
- Skin cancer continues to be the most common cancer diagnosed in the United States
- About 9,500 Americans are diagnosed with skin cancer every day, and about 3 million Americans have skin cancer
- About 1,600 people in the United States are diagnosed with an uncommon skin cancer called Merkel-cell carcinoma
- The advent of immunotherapy brings new hope for about half of the patients with this skin cancer