New results presented at the 2020 ASCO annual meeting show that patients with advanced or metastatic (spreading) cutaneous squamous-cell carcinoma (CSCC) who received the PD-1 inhibitor Libtayo (cemiplimab) lived much longer than patients who did not receive this PD-1 inhibitor.
Danny Rischin, MD, Director of the Department of Medical Oncology at Peter MacCallum Cancer Centre in Victoria, Australia, discussed the long-term results of an ongoing study with this drug.
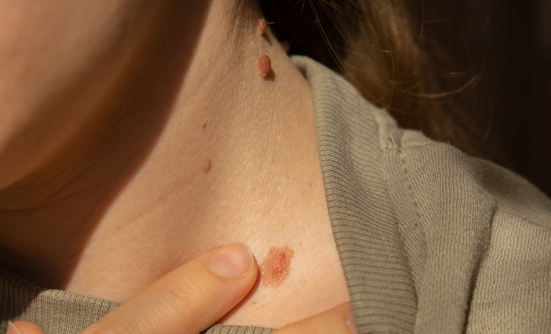
Skin cancer, or CSCC, is the second most common cancer in the United States, according to Dr. Rischin. In most cases, skin cancer is removed by surgery or by radiation, but in patients with advanced or metastatic CSCC, surgery is no longer an option. Because many patients with this type of cancer are diagnosed at a late stage, when the cancer is already locally advanced or metastatic, it can no longer be treated with surgery or radiation, which can cure it.
Information from previous studies of treatment for skin cancer showed that the average survival in patients with advanced or metastatic CSCC is about 15 months, according to Dr. Rischin.
Cemiplimab is a monoclonal antibody and is the only immunotherapy approved for patients with non-melanoma advanced or metastatic skin cancer. At the ASCO meeting, Dr. Rischin presented the new 3-year results of this phase 2 clinical trial, which showed that almost half (46.1%) of the patients who received cemiplimab therapy had a response to this immunotherapy.
“This analysis of Libtayo demonstrates increasing and impressive duration of response. Duration of response and overall survival were considerably longer than what has previously been described with other agents,” said Dr. Rischin.
Increased Survival
In the initial analysis of this study, after nearly 16 months of treatment with cemiplimab, the average duration of survival was not reached, meaning that patients were still responding to therapy and the cancer was not progressing.
Now the new analysis includes data for 3 years of follow-up that evaluated the impact of 3 years of cemiplimab treatment on survival and on the rate of patients with advanced or metastatic CSCC whose cancer has continued to respond to this treatment and was not progressing.
The study included 193 patients who were divided into 3 groups. Patients in group 1 had metastatic CSCC, and patients in group 2 had locally advanced disease; groups 1 and 2 received cemiplimab every 2 weeks. The patients in group 3 had metastatic CSCC and received cemiplimab every 3 weeks.
In group 1 in the original analysis in 2018, the rate of complete responses (no sign of cancer) was 6.8%, but a later analysis after an additional 1 year of follow-up, the complete response rate increased to 16.9%. And in the current analysis with 1 additional year of follow-up reported at the 2020 ASCO meeting, the response rate increased to 20.3%, showing improved survival with longer-term use of immunotherapy.
In group 2, no complete responses were seen at the original analysis, but at the additional 1-year follow-up, the complete response rate was 12.8%, which remained unchanged in this new analysis. In group 3, the complete response rate increased from 5.4% in the original analysis to 16.1% in this new 3-year follow-up analysis. These results suggest that cemiplimab should be the standard of care for patients with advanced or metastatic CSCC.
“The 3-year follow-up data demonstrate significant long-term outcomes with Libtayo, which is now standard-of-care for patients with advanced CSCC in many countries,” said Dr. Rischin.
“The Libtayo data on duration of response and overall survival provide new insights into the longer-term treatment of advanced CSCC….It is exciting to see the number of complete responses increase with longer follow-up, which reinforces the potential ongoing benefit of Libtayo treatment in this aggressive skin cancer,” Dr. Rischin said.
Long Duration of Response
Among patients who had a response, the responses lasted 6 months or more in 91% of the patients, and the estimated response rate for patients who are continuing to respond to treatment is 83.6%.
In addition, those who responded to cemiplimab therapy did so rather quickly. The time to response was 2 months in 46.1% of patients, and an additional 32.6% of patients had a response within 2 to 4 months. Among 89 of the patients with a response to therapy, the average time to a complete response was 11.2 months, according to Dr. Rischin.
The estimated average time without disease progression in all patients receiving cemiplimab was 18.4 months, and the average overall survival has not been reached for the total patient population in the study, meaning that many patients are still responding to therapy 3 years after starting treatment with this drug.
“This analysis indicates an increasing, clinically meaningful duration of response with Libtayo,” Dr. Rischin said.
Side Effects
Like all therapies, treatment with cemiplimab is associated with side effects, and most (99.5%) patients in the study had at least 1 side effect related to the treatment. However, most of the side effects were not severe (grades 1 and 2), and very few patients stopped therapy with this immunotherapy.
No new side effects were seen in the 3-year follow-up period, and no deaths were reported with this therapy, Dr. Rischin concluded.
Key Points
- Skin cancer, or CSCC, is the second most common cancer in the United States
- Many patients with skin cancer are diagnosed at a late stage, which can no longer be treated with curative surgery or radiotherapy
- New results of a 3-year study showed improved long-term survival of patients with CSCC with cemiplimab
- Cemiplimab is the first and only immunotherapy approved for patients with advanced or metastatic skin cancer