Skin cancer encompasses several types of cancer, including some types of melanoma. The most common type of non-melanoma skin cancer is basal-cell carcinoma. The second most common type is cutaneous squamous-cell carcinoma (or CSCC for short).
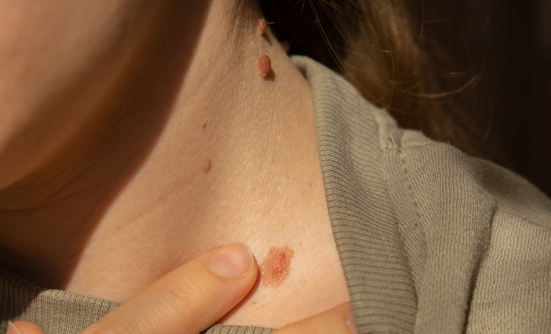
Cutaneous Squamous-Cell Carcinoma
In the past 3 decades, the number of cases of CSCC has been rising significantly every year in the United States. About 1.8 million cases of this type of skin cancer are diagnosed in the United States every year, making it one of the most common types of cancer in the country.
According to the Skin Cancer Foundation, the main cause of this type of skin cancer is sun exposure, so most cases of CSCC are found in areas that are exposed to the sun; however, CSCC can occur on any part of the body.
Therefore, the best way to prevent this very common skin cancer is to use sun protection or avoid sun exposure.
CSCC can be recognized by the appearance of scaly red skin patches, open sores on the skin, or thickened skin that looks like warts. Although early-stage CSCC can be treated successfully by surgery or radiation, if not diagnosed early and fully removed by surgery or radiation, it will usually grow to an advanced stage, or even spread to other parts of the body to become metastatic skin cancer, which can be deadly.
Immunotherapy for Skin Cancer
In 2018, the FDA approved the first immunotherapy, the PD-1 inhibitor Libtayo (cemiplimab-rwlc), for the treatment of patients with locally advanced or metastatic CSCC that cannot be removed by surgery or radiation. And in February 2021, Libtayo became the first immunotherapy to be approved by the FDA for the first-line treatment of patients with advanced basal-cell carcinoma.
Now results of a new study presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting showed that immunotherapy with Libtayo is also safe and effective for the treatment of patients with advanced CSCC who are immunocompromised or immunosuppressed.
Immunocompromised People
Immunocompromised or immunosuppressed people are people whose immune system is weakened because of certain medical conditions or because they have an auto-immune condition, such as arthritis or psoriasis, and they are taking immunosuppressant medications that intend to reduce the function of their immune system. Immunocompromised people have a reduced ability to fight infectious diseases, because their weak immune system does not provide enough protection against infections.
This became a major concern in the past year in relation to COVID-19, raising the question of how effective the COVID-19 vaccines are in patients with cancer, many of whom may be immunocompromised. Some of the medical conditions that weaken the immune system include a cancer diagnosis, diabetes, undergoing a stem-cell or organ transplant, taking certain anti-cancer drugs, or taking immunosuppressive drugs to treat auto-immune diseases.
Immunocompromised people are also at increased risk of several medical conditions, including skin cancer and other types of cancer.
Immunotherapy Effective in Immunocompromised Patients with CSCC
The new study results presented at the ASCO meeting showed that immunotherapy is beneficial in immunocompromised or immunosuppressed patients with the CSCC. These new data have the potential to change the standard of care for immunocompromised patients with CSCC.
Patients who are immunocompromised or immunosuppressed are at increased risk of solid tumor or skin cancer. The risk of non-melanoma skin cancer in these people is increased by as much as 10 times and up to 250 times compared with people who are not immunocompromised.
Until recently, no good information was available about the safety and effectiveness of immunotherapy in immunocompromised patients, mostly because such patients are often excluded from clinical trials of immunotherapies, including the newer immune checkpoint inhibitors such as Libtayo. Yet clinical trials are the main way that researchers find information about new cancer therapies.
As noted earlier, the PD-1 inhibitor Libtayo has already been approved by the FDA for the treatment of patients with advanced CSCC whose cancer cannot be removed by surgery or radiation, but it was not known if it could also benefit immunocompromised or immunosuppressed patients with this type of skin cancer. To answer this question, the new study of Libtayo immunotherapy in patients with advanced CSCC also included immunocompromised and immunosuppressed patients.

“The safety, tolerability, and effectiveness of cemiplimab [Libtayo] in certain immunosuppressed or immunocompromised patients with advanced CSCC appear to be consistent with those observed in clinical trials that excluded these patients,” said Guilherme Rabinowits, MD, a Hematologist/Oncologist at Miami Cancer Institute/Baptist Health South Florida, Miami, at the recent ASCO meeting.
“Further follow-up and additional data would add to our general understanding of safety and effectiveness of anti-PD-1 therapy in these patient populations,” Dr. Rabinowits added.
Good Response to Immunotherapy
Dr. Rabinowits discussed the results of the study that evaluated the effectiveness, safety, quality of life, and survivorship in patients with advanced CSCC who received immunotherapy with Libtayo. All patients with advanced CSCC, including immunocompromised or immunosuppressed patients, were qualified to be included in this study.
Patients were identified as immunocompromised or immunosuppressed if they had any condition that may weaken their immune system, including a transplant, leukemia, lymphoma, or multiple myeloma, among others. At the ASCO meeting, Dr. Rabinowits focused on the patients who were immunosuppressed or immunocompromised and had advanced-stage CSCC.
By March 2021, 138 patients were enrolled in the study, and 30 of them were immunocompromised: 6 patients had received a transplant, 14 patients had a blood cancer, and 10 patients had an auto-immune disorder. All patients with advanced CSCC received intravenous Libtayo every 3 weeks as the standard of care. The average length of treatment was 21 weeks, but 9 patients received Libtayo for at least 48 weeks.
Overall, nearly half (45.5%) of the patients had a response to Libtayo therapy, according to Dr. Rabinowits. In addition, although 8 patients were excluded from the final analysis, 6 of these patients also had a response to the immunotherapy.
In terms of side effects, 1 patient discontinued Libtayo therapy because of a side effect and 8 patients discontinued treatment for other reasons.
Although 4 deaths were reported during the study, none was related to Libtayo therapy, Dr. Rabinowits said. As expected with immunotherapy, 6 patients who received Libtayo had immune-related side effects, including fatigue, skin itching on the forehead and chest, elevated laboratory blood levels, reduced lymphocytes, and hypothyroidism.
In addition, 1 patient had acute kidney failure, so Libtayo treatment was stopped, and the skin cancer returned. The patient is now on dialysis, and the patient is planning to resume Libtayo treatment for the skin cancer.
Patient Resources
American Cancer Society
www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/low-blood-counts/infections/why-people-with-cancer-are-at-risk.html
CDC/Centers for Disease Control and Prevention
www.cdc.gov/parasites/crypto/gen_info/infect_ic.html
Skin Cancer Foundation
www.skincancer.org/skin-cancer-information/squamous-cell-carcinoma/