When a breast cancer diagnosis is made by a pathologist, the type of breast cancer is determined by cellular appearance, staining results, and validated procedures. The type of breast cancer is one factor that a doctor needs to know when a treatment plan is designed. Other prognostic factors paint a specific picture of the breast cancer, such as tumor size, lymph node involvement, tumor grade, estrogen receptor status, progesterone receptor status, and HER2-neu status. Many times, this additional information helps the team make a specific treatment plan for the patient, such as surgery, radiation therapy, endocrine therapy, or chemotherapy.
But in certain cases, it is not evident whether chemotherapy should be offered for breast cancer treatment. The dilemma is that if chemotherapy is administered to a patient when the disease is unlikely to recur, it is reasonable to expect that the side effects and possible complications would outweigh any benefit to the individual. To answer the question of whether to treat with chemotherapy, genomic tests have been developed that look at a sample of a breast cancer tissue and analyze how likely it is that the cancer cells will grow and spread in the future if not treated.
Genomic tests involve technology that determines how active certain genes are, and how this activity level affects the behavior of the cancer. The tests can be done on a sample of preserved tissue that was removed from the breast during biopsy or surgery. This is very different from genetic testing, which is completed on a sample of blood, saliva, or other tissue to see if an individual has an abnormal change or mutation in a gene that is related to a higher risk of breast cancer. Genomic testing explores recurrence of cancer, and genetic testing investigates the risk of ever getting cancer.
The 2 most common FDA-approved genomic tests used to determine chemotherapy benefit are Oncotype DX and MammaPrint. They work by going inside the cancer cell and exploring its DNA-specific genes to give a more unique view of a breast cancer. Each method helps oncologists and patients select the right treatment, optimize the care, and avoid unnecessary therapy.
Oncotype DX testing has involved over 63,000 early-stage breast cancer patients from 4 large independently run studies (SEER Registry, TAILORx, Clalit, and West German Study Group PlanB) in more than 90 countries. Early-stage breast cancer means the cancer is limited to the breast and/or regional lymph nodes. The process examines the activity of 21 genes in the breast cancer tissue to determine the likelihood of a chemotherapy benefit, how effective the treatment will be with hormonal therapy alone, as well as the chance of cancer recurrence. The test is designed for all newly diagnosed patients with early-stage (stage I, II, or IIIa) breast cancer with node-negative disease or with 1 to 3 positive nodes. They must have estrogen receptor–positive (ER+) and HER2-negative disease.
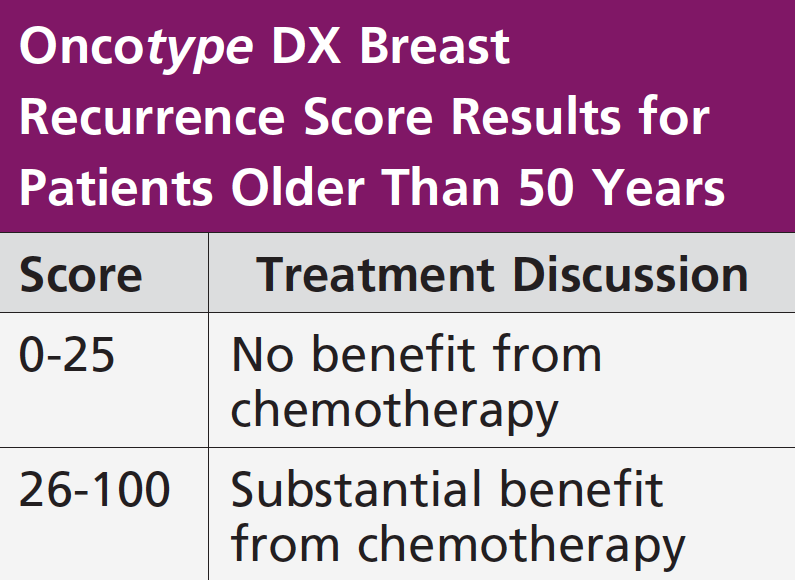
The Oncotype DX Breast Recurrence Score is based on a continuous scale from 0 to 100, with the higher numbers reflecting an increased risk of distant recurrence and a higher likelihood of benefit from chemotherapy. It also shows a numerical estrogen receptor score to help assess the magnitude of hormonal therapy benefit. The score is a visual tool for the treatment team to use in discussing treatment options with the patient.
The earliest studies showed that 99% of patients with a low Oncotype DX score (18 or lower) who were primarily treated using hormonal therapy alone, without chemotherapy, were cancer free after 5 years. Patients with a high recurrence score (greater than 31) did well with chemotherapy. So, there was an answer for the 2 extreme scores, but concern was for patients who fell in the intermediate-risk group (19-30). This cohort had no data to support whether they should have chemotherapy. Therefore, a large, randomized, adjuvant breast cancer treatment trial called TAILORx was conducted and definitively identified the 70% of women with intermediate Oncotype DX scores who will not benefit from chemotherapy and the 30% for whom chemotherapy can be lifesaving. The scores reported on 2 age-groups—those older than 50 years and those younger than 50 years.
The Oncotype DX test is included in major cancer guidelines worldwide as standard of care for women with early-stage breast cancer. Most US insurance carriers cover the Oncotype DX test for eligible patients. Genomic Health has a platform called Genomic Access Program to help navigate insurance and other payment options for Oncotype DX tests.
To answer the question of whether to treat with radiation therapy, Oncotype DX is also used for patients with ductal carcinoma in situ (DCIS) or stage 0 breast cancer. Again, the focus is on selecting the best treatment options based on an individual patient’s risk for a local recurrence with either DCIS or invasive breast carcinoma. Any patient who has been diagnosed with DCIS and treated with a lumpectomy or undergone a core biopsy is eligible for this analysis. The Breast DCIS Score test is performed on a tumor sample after a biopsy or surgery. It analyzes 12 cancer-related genes to predict the 10-year risk of local recurrence and conveys a baseline score to help the radiation oncologist and patient decide if radiation therapy will be beneficial.
Just like the Oncotype DX Breast Recurrence Score, the Breast DCIS Score report includes a result on a range from 0 to 100, with high-risk estimates having a greater absolute benefit from radiation therapy. This test is covered by Medicare, but private insurance coverage varies among companies (see chart on next page).
MammaPrint testing has involved 208 patients in a study called RASTER conducted in 16 community-based clinics in the Netherlands and over 6693 breast cancer patients enrolled from 112 participating institutions in 9 European countries via a prospective, phase 3 study called MINDACT. The process explores 70 genes to answer the questions of breast cancer recurrence. The test is designed for all newly diagnosed patients with early-stage breast cancer (stage I or II), tumor size up to 5 cm, and either lymph node–negative or with 1 to 3 positive lymph nodes. The patient does not have to be ER+, and this differentiates MammaPrint from other genomic assays in use today.
The MammaPrint report categorizes the patient as low risk or high risk. Low risk is defined as a 1.3% chance that cancer will recur within 5 years, and high risk is defined as a 11.7% risk of recurrence. The National Comprehensive Cancer Network recognizes the test for prognosis of breast cancer in women of all ages, and the American Society of Clinical Oncology recommends this genomic test for women with lymph node–positive disease. Medicare reimburses for this test, but private insurance coverage varies among companies. MammaPrint has a customer care team to help patients overcome the barrier of insurance coverage and payment support.
Not every breast cancer diagnosis requires an Oncotype DX or MammaPrint analysis for a treatment decision. The prognostic factors, such as estrogen receptor, progesterone receptor, and HER2 scores, as well as age, tumor grade, and stage, and additional health concerns will guide a personalized treatment decision. Genomic testing is one tool an oncologist may use to assess treatment options with the patient.
Sources
- Genomic Health. Oncotype DX for Breast Cancer. www.genomichealth.com/en-US/Oncotype_iq_products/oncotype_dx/Oncotype_dx_breast_cancer. Accessed March 28, 2019.
- Agendia. MammaPrint: a test to predict risk of breast cancer recurrence. www.agendia.com/our-tests/mammaprint. Accessed March 28, 2019.