Quality of Life | Final Thoughts | Appendix
One of the most important meetings of physicians and other healthcare professionals who specialize in hematologic malignancies, including multiple myeloma, is held each December in a major US city by the American Society of Hematology (ASH). ASH is the world’s largest professional society concerned with the causes and treatments of blood disorders. More than 28,000 physicians, nurses, pharmacists, and other stakeholders in cancer care attended this year’s meeting, which took place in San Diego, California, December 1-4, 2018.
This publication features research in the care of patients with multiple myeloma that was presented at the 2018 ASH Annual Meeting, including clinical trial results and studies that focus on quality of life. Each section describes a specific presentation and includes the conclusions reached by the researchers who presented the data. We also share insights about these research projects from people who are living with multiple myeloma and are patient advocates for others with the disease, including Jack Aiello, Barbara Kavanagh, MSW, LCSW, and James Omel, MD. These patient opinion leaders—who have had multiple myeloma themselves (or have a family member with the disease) and who are actively engaged in outreach to other patients, their caregivers, and families—explain why the research efforts are important, as well as what these findings may mean, both for patients with multiple myeloma and for their physicians and healthcare teams.
Newly Diagnosed
Transplant Status Does Not Affect Selection of Induction Regimens for Patients with Newly Diagnosed Multiple Myeloma in the INSIGHT MM Trial
The introduction of several novel agents and regimens for newly diagnosed multiple myeloma and relapsed/refractory multiple myeloma has improved outcomes while increasing the complexity of treatment selection and disease management. The real-world effectiveness of many regimens based on novel agents remains to be elucidated.
INSIGHT MM, the largest global, prospective, observational study of multiple myeloma conducted to date, aims to understand the characteristics of patients with newly diagnosed and relapsed/refractory multiple myeloma, treatment patterns, and clinical outcomes, as well as regional variations. It is enrolling approximately 4200 adults around the world: Europe, United States, Latin America, and Asia. Patients will be followed prospectively for at least 5 years. Data will be collected from hospital and clinic records for each patient at the time of study enrollment and then every 3 months.
 |
“I think that now, if you are in the middle of the country and you have a community oncologist, the good news is that there is more and more understanding among community oncologists about multiple myeloma. And I still think it is worth traveling to the nearest large city from where you live to get a second opinion and add a multiple myeloma expert on your team.”—Jack Aiello |
At the time of data cutoff for this analysis, 1056 patients had been enrolled from 14 countries. Most patients were from Europe (47%) or the United States (34%), whereas fewer patients were from Latin America (11%) and Taiwan (8%).
Median age at enrollment in INSIGHT MM was 64 years (range, 32-89 years), with 13% of patients aged 75 years or older. Overall, 62% of patients were treated for their multiple myeloma at academic centers or university hospitals, and 38% received care in community settings, such as offices, clinics, and community hospitals. There were regional differences in the setting of care: relatively more patients with multiple myeloma were treated at academic centers in Europe and Taiwan compared with the United States and Latin America. Almost all (87%) patients were not treated in clinical trials.
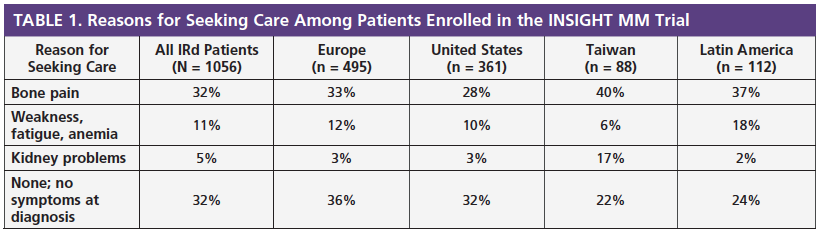
At ASH 2018, researchers also presented data that profiled patients with multiple myeloma in terms of the most common reasons for seeking care and the treatments received. Table 1 summarizes the most common reasons for patients seeking care. At diagnosis, 27% of all patients had physician-reported International Staging System (ISS) stage I multiple myeloma, 26% had physician-reported ISS stage II disease, and 31% of patients had physician-reported ISS stage III disease.
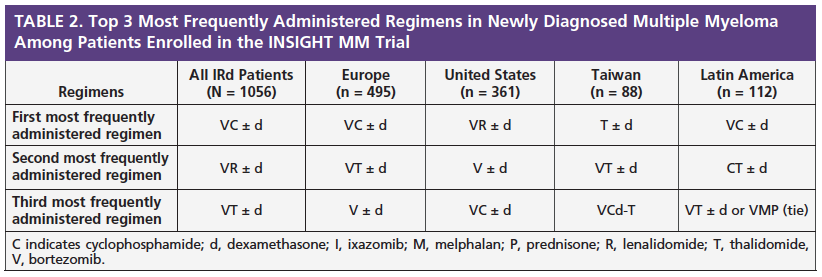
The most frequently administered initial treatment regimens for patients who were newly diagnosed with multiple myeloma are shown in Table 2. Overall, 66% of patients received a triplet (3-drug) regimen and 20% received a doublet (2-drug) regimen. Bortezomib (Velcade)-based regimens were the most frequently used.
There were regional differences in treatment selection. Among the immunomodulatory drugs (IMiDs), thalidomide (Thalomid) was more commonly prescribed in Europe, Taiwan, and Latin America, and lenalidomide (Revlimid) was more commonly used in the United States.
At the time of data cutoff, results were available for 236 patients who underwent autologous stem-cell transplantation (ASCT) after their induction treatment. Of these patients, 42% were treated in Europe, 42% in the United States, 11% in Taiwan, and 4% in Latin America. Their median age was 60 years, with 25% of patients aged 65 years or older. Of these patients who received ASCT, 64% did so at an academic center.
Proteasome inhibitors and IMiDs remain the global backbones of multiple myeloma therapy, with bortezomib-based regimens most commonly used in newly diagnosed multiple myeloma patients, regardless of intended transplant status. The induction regimens that were given most frequently to patients who were eligible for ASCT were bortezomib plus cyclophosphamide (Cytoxan) with or without dexamethasone (VC ± d), bortezomib plus lenalidomide with or without dexamethasone (VR ± d), and bortezomib plus thalidomide with or without dexamethasone (VT ± d).
The preliminary results from INSIGHT MM have uncovered differences in presentation and treatment of multiple myeloma among the regions of the world represented in the study. For example, higher proportions of patients receive ASCT in the United States and Europe than in Taiwan and Latin America. This may reflect differences in healthcare systems and access to multiple myeloma treatments in these participating countries. Future studies will evaluate the impact of these regional variations on patient outcomes, including overall survival.
Source
- Usmani SZ, Hungria VTM, Leleu X, et al. Transplant status does not impact the selection of induction regimens for newly diagnosed multiple myeloma (NDMM) patients (pts) in the INSIGHT MM prospective, observational study. Presented at the 2018 ASH Annual Meeting; December 2, 2018; San Diego, CA. Abstract 3289.
Carfilzomib/Lenalidomide/Dexamethasone versus Bortezomib/Lenalidomide/Dexamethasone in Patients with Newly Diagnosed Multiple Myeloma: Results from the CoMMpass Study
Triplet (3-drug) regimens that include a proteasome inhibitor (PI), such as bortezomib (Velcade), and an immunomodulatory drug (IMiD), such as lenalidomide (Revlimid), are typically used to treat patients who are newly diagnosed with multiple myeloma. In the United States, 2 triplet combination regimens—carfilzomib (Kyprolis)/lenalidomide/dexamethasone (KRd); and bortezomib/lenalidomide/dexamethasone (VRd)—are recommended for the treatment of newly diagnosed multiple myeloma by the National Comprehensive Cancer Network. However, there is no information about how these 2 regimens compare directly in terms of efficacy and tolerability in patients with newly diagnosed multiple myeloma.
| “The importance of this head-to-head comparison is confirmation that a triplet combination approach of a PI, IMiD, and dexamethasone is a very effective initial treatment for multiple myeloma. The KRd triplet had a higher EFS, but I would not make that the sole basis for my treatment decision. It is important to also consider quality of life (QoL). Carfilzomib must be given by an intravenous infusion, compared to a quick subcutaneous injection of bortezomib, which gets me out of the treatment center much faster (a better QoL). Sitting in an infusion chair does not qualify as “quality” time. My doctor and I review studies such as this, discuss treatment options, and then I decide what the treatment should be. Ultimately every patient needs to make his/her own treatment decision. Their doctor is a teacher/explainer, but the patient is the one who must make the final decision for treatment plans.”—James Omel, MD |  |
At ASH 2018, researchers reported the results of a prospective study of efficacy and preliminary tolerability data for patients who received KRd or VRd as initial therapy for multiple myeloma in the Multiple Myeloma Research Foundation–funded CoMMpass study.
CoMMpass, a prospective observational study, has been underway since 2011. Its database currently includes more than 1100 patients, all of whom were enrolled within 30 days of initiating therapy for newly diagnosed multiple myeloma. These patients’ first-line therapy was chosen by their physicians and had to include a PI and/or an IMiD.
Among the 609 evaluable patients enrolled in CoMMpass, 149 received KRd and 460 received VRd as first-line therapy. Researchers evaluated the effectiveness of each treatment regimen, as well as reasons for treatment discontinuation. In this study, the 149 KRd patients were matched to the 149 VRd patients based on their age, gender, risk status, and kidney function. The median age of these patients was 58 years, most (63%) were men, and most had International Staging System (ISS) stage I (~50%) or ISS stage II (40%) disease. Event-free survival (EFS), which was defined as the time from the start of treatment until disease progression, initiation of new therapy, or death, was the prespecified primary end point.
After follow-up of approximately 12 months for KRd and approximately 49 months for VRd, 12-month rates of EFS were 90% for KRd and 78% for VRd, which was a statistically significant difference in favor of KRd. After 12 months, 80% of KRd patients and 64% of VRd patients had a partial response or better, which was also a statistically significant difference in favor of KRd. Among patients who received KRd, 23% of patients achieved a complete response or better, compared with 15% of patients who received VRd (statistically significant). In both groups of patients, approximately 5% discontinued treatment because of side effects.
These research findings are consistent with previous single-arm studies that suggest that the KRd triplet is not only effective but potentially more effective than VRd for patients with newly diagnosed multiple myeloma.
Source
- Landgren O, Siegel DS, Auclair D, et al. Carfilzomib-lenalidomide-dexamethasone versus bortezomib-lenalidomide- dexamethasone in patients with newly diagnosed multiple myeloma: results from the prospective, longitudinal, observational CoMMpass study. Presented at the 2018 ASH Annual Meeting; December 3, 2018; San Diego, CA. Abstract 799.
Relapsed/Refractory
Results from a Phase 2 Study of Isatuximab as a Single Agent and in Combination with Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma
Isatuximab is a monoclonal antibody that is in clinical development for patients with multiple myeloma who have received more than 2 prior therapies. This drug, which is in the same class as daratumumab (Darzalex), targets CD38-expressing tumor cells. Although not yet approved by the US Food and Drug Administration (FDA), isatuximab has been shown in early clinical studies to be effective in the treatment of patients with relapsed or refractory multiple myeloma (Martin TG et al. J Clin Oncol. 2014;32:15 suppl. Abstract 8532).
 |
“If I had an option of a proven effective treatment option which I had never used before, versus a new treatment, I would go with what has been proven. The only thing that would prompt me to go with the new therapy would be solid clinical trial data showing a significant improvement over my currently available treatment choices.”—James Omel, MD |
At ASH 2018, researchers shared results of an ongoing phase 2 clinical trial of isatuximab in patients with relapsed or refractory multiple myeloma. This study occurred in 2 stages and had 2 key objectives. In the first stage of the trial, the goal was to select the optimal dose of isatuximab. In the second stage, which is ongoing, researchers are assessing the efficacy and safety of isatuximab given alone or in combination with dexamethasone to patients with relapsed or refractory multiple myeloma.
The dose of isatuximab that was selected for the second stage of the trial was 20 mg/kg given each week for cycle 1 followed by 20 mg/kg given every 2 weeks. Isatuximab is administered as an intravenous (IV) infusion.
The second stage of the trial enrolled 165 patients with multiple myeloma who had previously received an immunomodulatory drug (IMiD) and a proteasome inhibitor (PI). Patients received isatuximab (20 mg/kg IV on days 1, 8, 15, and 22 per week of cycle 1, followed by 20 mg/kg IV on days 1 and 15 every 2 weeks of subsequent cycles), either alone or combined with dexamethasone.
The median age of the patients who enrolled in this trial was 67 years (range, 37-85 years). These patients had received a median of 4 previous lines of treatment (range, 2-11 lines) before enrolling in the trial and receiving isatuximab. Patients received a median of 5 cycles of isatuximab (range, 117 cycles) and stayed on treatment for a median of 22 weeks (range, 1-69 weeks). Almost two-thirds (64%) of patients stopped taking isatuximab because of disease progression (52%), adverse events (9%), or personal preference (4%).
Data analysis from this ongoing trial confirms that isatuximab, used alone or in combination with dexamethasone, is effective in heavily pretreated patients with relapsed or refractory multiple myeloma. Specifically, researchers showed that the addition of dexamethasone increased response rates associated with isatuximab from 26% (isatuximab alone) to 44% (isatuximab plus dexamethasone). Median progression-free survival (PFS) was also longer for patients who received the combination of isatuximab and dexamethasone compared with those who received isatuximab alone (median PFS: 4.9 months vs 9.3 months, respectively).
Isatuximab was generally well- tolerated. The addition of dexamethasone did result in steroid-related side effects, but these were considered manageable.
Researchers concluded that isatuximab is effective and safe in patients with multiple myeloma who have relapsed after treatment with a PI, such as bortezomib (Velcade), and an IMiD, such as lenalidomide (Revlimid). Isatuximab may be one of the next treatments to receive FDA approval for the treatment of multiple myeloma.
Source
- Dimopoulos MAD, Bringhen S, Anttila P, et al. Results from a phase II study of isatuximab as a single agent and in combination with dexamethasone in patients with relapsed/refractory multiple myeloma. Presented at the 2018 ASH Annual Meeting; December 1, 2018. Abstract 155.
Dynamic Changes in International Staging System as a Predictor of Survival Outcome in Patients with Advanced Multiple Myeloma
After a patient is diagnosed with cancer, physicians perform tests to determine whether the cancer has spread and if so, how far. This process, called staging, helps physicians learn how serious the cancer is and identify treatments that may be most valuable. Physicians also use cancer stage to determine a patient’s risk for progression and likely survival (ie, prognosis).
Multiple myeloma is staged using the revised International Staging System (ISS). The ISS uses 4 factors to determine whether a patient’s cancer is ISS stage I, II, or III:
- Amount of albumin in the blood
- Amount of beta-2 microglobulin in the blood
- Amount of lactic dehydrogenase (LDH) in the blood
- Specific gene abnormalities of the cancer (cytogenetics).
Recent studies suggest that physicians who use the ISS to determine risk for progression should consider how each patient’s multiple myeloma evolves. At ASH 2018, researchers discussed how patients’ ISS stage can shift over time. They also described the way in which these changes may affect outcomes for patients with multiple myeloma.
| “It is not typically done and, to my knowledge, it was always said that ISS staging is useful at diagnosis. But it is not so useful afterwards. It is not so useful when you have relapsed because treatment you have taken can affect your numbers. What can be important is to get a bone marrow biopsy when you have relapsed, to see whether your myeloma cell has cloned itself to have different high-risk factors than maybe it did when you were initially treated. ISS staging is just a tool that can be used to say that if you are diagnosed at stage I, then you, on average, have a better chance for a longer survival than you were diagnosed at stage III.”—Jack Aiello |  |
This research effort included 417 patients with multiple myeloma from a medical records database managed by a company called Flatiron. Data for patients in the Flatiron database who have received at least 2 lines of therapy for multiple myeloma and who had information about their ISS at 2 time points (time of diagnosis and time of initiating second-line therapy) were included in the analysis.
Using specific information in the patient records, researchers estimated how ISS stage, both at the time of diagnosis and atthe time of initiating second-line therapy, impacted mortality rates. In the subgroup of patients in ISS stage III at the time of diagnosis, the investigators used data analysis models to learn factors that predict a downward shift from ISS stage III to ISS stage I or II at the time patients started second-line therapy.
Patients in the study were aged 70 years (median age) and 59% were men. At the time of diagnosis with multiple myeloma, 30%, 37%, and 33% of the study group were classified as ISS stage I, II, and III, respectively. The mortality rates associated with these stages were 12, 11, and 24 deaths per 100 person-years, respectively. At the time they started second-line therapy for their multiple myeloma, 47%, 34%, and 20% of these patients had ISS stage I, II, and III disease, respectively, with mortality rates of 7, 19, and 39 deaths per 100 person-years, respectively, at this time point.
Among patients who were ISS stage I at diagnosis, approximately 25% had shifted to higher ISS stages when they started second-line therapy. These patients had a higher mortality rate (26 per 100 person-years) than patients who remained in ISS stage I (8 per 100 person-years).
For patients who were in ISS stage II at diagnosis, 43% were still in stage II when they started second-line therapy, whereas 46% had moved down to ISS stage I. Their mortality rates were 20 and 5 per 100 person-years, respectively.
Among patients in ISS stage III at diagnosis, 58% had moved down to lower stages when they started second-line therapy. Mortality rates were 10 and 21 per 100 person-years for patients who moved down to ISS stage I and stage II, respectively, at the time of initiating second-line therapy. The mortality rate was 40 per 100 person-years for patients who remained in ISS stage III when they started second-line therapy.
In the subgroup of patients who were ISS stage III at diagnosis, strong predictors for shifting down to lower stages were younger age and serum creatinine of 2 mg/dL or less at time of diagnosis. Sex, race, and the presence of specific cytogenetic abnormalities did not predict a downward shift to lower ISS stages.
Large changes in ISS stage were observed in patients with multiple myeloma as they advanced through more lines of therapy. Changes in ISS stage were also associated with survival outcomes. For example, a downward shift to ISS stage I was associated with substantial improvements in overall survival. Patients who moved up in terms of ISS stage or who remained in higher stages despite treatment had poorer outcomes, especially those who remained in ISS stage III. These results suggest that reevaluating ISS stage at the time of change in line of therapy can help physicians better predict survival outcomes for their patients with multiple myeloma.
 |
“I would not find ISS re-staging useful. When I need my next therapy, my doctor and I will together choose the best available treatment combination. I find the information interesting, with the outcomes just as would be expected, but of little practical use for me personally. My physician does not periodically reevaluate my ISS stage. I have had a prolonged remission. I get quarterly evaluations of my serum free light chains and a quantitative serum immunoglobulin determination each year, but do not routinely check LDH, albumin, or beta-2 microglobulin levels (which are all part of ISS staging). If and when multiple myeloma becomes active again and I must begin my fifth line of therapy, I will want to know my genomic profiling results more than my ISS results.”—James Omel, MD |
Source
- Hong J-L, Crossland V, Galaznik A, et al. Dynamic changes in international staging system as a predictor of survival outcome in patients with advanced multiple myeloma. Presented at the 2018 ASH Annual Meeting; December 3, 2018; San Diego, CA. Abstract 4438.
Sociodemographic Features and Societal Perspective of Patients with Relapsed/Refractory Multiple Myeloma in Spain: Interim Analysis of the CharisMMa Study
Patients with multiple myeloma often receive several courses or lines of treatment. Periods of remission are followed by relapse and a new line of therapy. There are many treatments for patients with multiple myeloma who relapse, which is often thought to be helpful since every patient with multiple myeloma is different. People with multiple myeloma who relapse may or may not have symptoms of the disease, they may be younger or older, they may have other medical conditions, and they may have specific disease features that others do not.
In light of all of these differences, selection of treatments for patients with multiple myeloma who relapse can be challenging. Physicians and their patients are tasked with balancing expected benefits of each treatment with possible side effects, healthcare resource use, and the treatment’s impact on health-related quality of life. Hematologists should consider the patient as a whole when making choices among treatments.
The CharisMMa study, an observational, cross-sectional, multicenter study conducted in Spain, was initiated to learn more about people with relapsed or refractory multiple myeloma. Thirty public hospitals in Spain provided information about their patients with multiple myeloma who required treatment at any disease relapse.
Researchers presented an interim analysis at ASH 2018. Their goal was to learn more about patients’ social and demographic characteristics and information about their disease. A total of 169 patients with relapsed or refractory multiple myeloma had enrolled in this study through June 2018 (total enrollment is expected to be 350 patients). On average, information about the patients was collected approximately 2 months after their last relapse.
The median age of patients at the time of their study visit was 69 years (range, 57-75 years). More than half (55%) of the patients were male. Most people lived in cities (72%) and with their families (88%). They were more likely to be retired (64%) and nondependent on others for daily care (73%). Patients had received a median of 2 previous treatments for myeloma (range, 1-3 treatments). Approximately half (57%) of them had undergone a stem-cell transplant (98% autologous). Their International Staging System status was balanced among the 3 stages: 36% stage I, 36% stage II, and 28% stage III. Most (74%) presented with typical symptoms of multiple myeloma, such as bone lesions (66%). Most (68%) of these patients suffered from another noncancer condition, such as heart disease (48%), diabetes (23%), or nerve damage (22%).
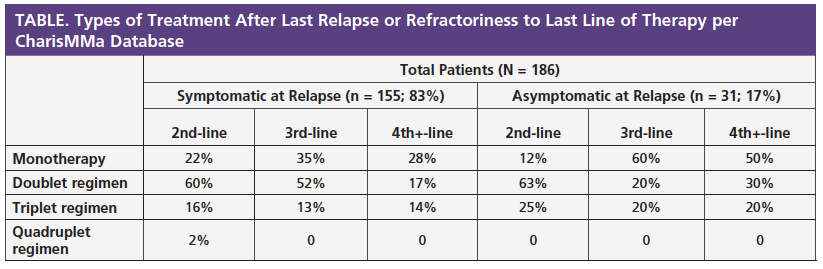
Patients who had symptoms of multiple myeloma at the time they relapsed were treated mainly with doublet (2-drug) regimens, both in the second-line setting (60%) and in the third-line setting (52%). The small group of patients who relapsed with multiple myeloma and who did not have symptoms were more likely to be treated with doublet regimens in the second-line setting (63%) and with a single agent in third-line regimens (60%) (Table).
CharisMMa also collected information about social factors for patients with multiple myeloma. On average, patients with the disease live 24 kilometers (15 miles) from their hospital or cancer center. They visit their doctor an average of 5 times each month, with 4 of these visits related to their treatment. In most (90%) of the visits, patients had a caregiver with them who works outside the home (57%).
After analyzing these data from CharisMMa, researchers determined that the wide variety of characteristics in people with multiple myeloma should be a major factor in the management of this malignancy. Different types and levels of resources are needed throughout each patient’s journey, and, as such, physicians should work with the patient and his or her family to develop an individual approach that maximizes clinical outcomes, facilitates appropriate resource use, and reduces indirect costs associated with multiple myeloma.
Source
- Ocio EM, Rosinol L, Grande, M, et al. Socio-demographic features and societal perspective of relapse and/or refractory multiple myeloma (RRMM) patients in Spain: an interim analysis of CharisMMa study. Presented at the 2018 ASH Annual Meeting; December 1, 2018; San Diego, CA. Abstract 2300.
Patient Preferences for Multiple Myeloma Treatment: Interim Analysis of a Discrete Choice Experiment
As more treatments become available for patients with relapsed or refractory multiple myeloma, information about patient preferences become more important in treatment selection. The US Food and Drug Administration, insurance companies, and individual physicians now pay attention to patient assessments of their experiences with new treatment regimens. This information is often referred to as “patient-reported outcomes” or PROs. PROs are valuable as supplements to traditional measures of drug effectiveness, safety, and tolerability, such as measurements of response and overall survival.
Although many new treatments for multiple myeloma have demonstrated longer time to progression and improved overall survival, these treatments vary with respect to safety, tolerability (side effects), administration methods (oral or intravenous), and dosing schedules. Patients and their care team can consider different trade-offs as they select among treatments. To learn more about patient preferences among currently available treatments for relapsed or refractory multiple myeloma, an analysis of an ongoing clinical trial was conducted and reported at ASH 2018.
In this preference study, adults who were diagnosed with multiple myeloma and who had received or were currently receiving first-line, second-line, or third-line therapy completed an online survey. These patients were recruited from targeted panels, advocacy partnerships, patient communities, and physician referrals during May and June 2018, with the number of survey respondents targeted at 200.
The survey was designed using a “discrete choice” approach, which asks patients to state their preferences and willingness to accept trade-offs among a series of hypothetical treatments with varying levels of specific attributes. For example, one treatment might offer a higher response rate but require a long intravenous infusion every week. Another treatment might have lower response rates but is given orally once a week.
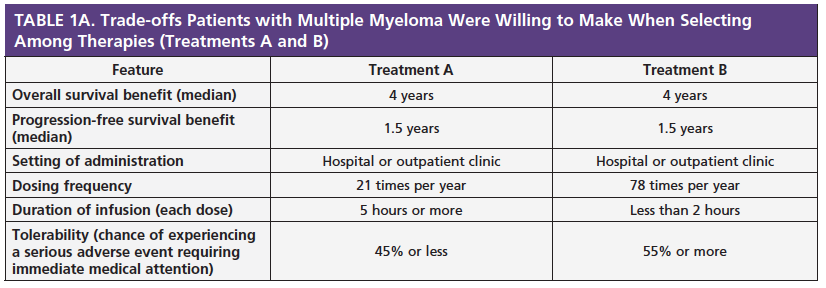
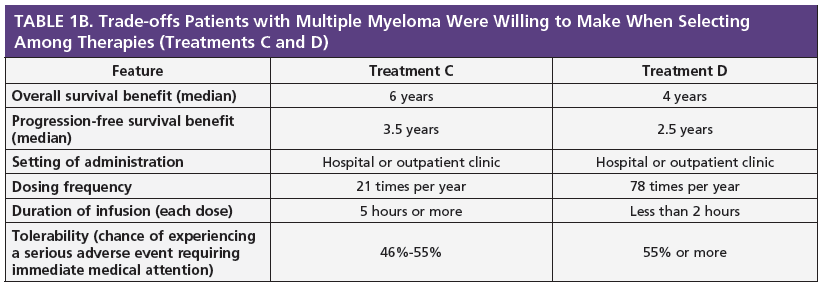
Patients were asked to rate the importance of each treatment attribute, such as response rate and whether it is oral or intravenous, using a 5-point scale, ranging from 1 (very bad) to 5 (very good). They were then asked to pick which treatment regimen they preferred between 2 different hypothetical treatment regimens. The goal was to identify trade-offs that patients were willing to make when selecting among treatments (see examples in Table 1A and 1B).
At ASH 2018, researchers presented an interim analysis with data from 74 people with multiple myeloma. Their average age was 63 years, and 51% of patients were male. Their average time since diagnosis was 70 months (approximately 6 years). Of the 74 patients, 25 were receiving first-line therapy, 25 were receiving second-line therapy, and 24 were receiving third-line therapy.
After analyzing the survey responses, researchers learned that patients who were receiving first- or second-line therapy considered overall survival (efficacy) more important than those who were receiving third-line treatment. Tolerability was also more important for patients who were in earlier lines of therapy.
When asked to choose between treatments with similar efficacy outcomes (treatments C and D in Table 1B), 92% of patients preferred the treatment with a lower frequency of dosing over a 1-year period, even if it required a longer infusion time (more than 5 hours) compared with a treatment that was given more often with a shorter infusion time (less than 2 hours).
These results suggest that patient preferences among multiple myeloma treatments vary as the patient receives more treatments. Additionally, dosing frequency is relatively more important than the time required for treatment administration. Patients seem to consider treatment options holistically: convenience is not simply less “chair time” but also includes frequency of outpatient visits.
Source
- McKay C, Maiese EM, Chiarappa J, et al. Patient preferences for multiple myeloma (MM) treatment: interim analysis of a discrete choice experiment. Presented at the 2018 ASH Annual Meeting; December 2, 2018; San Diego, CA. Abstract 3586.
Treatment Preferences for Relapsed/Refractory Multiple Myeloma: Are Patients Willing to Trade Efficacy for Tolerability?
As more agents and regimens are approved by the US Food and Drug Administration for relapsed and refractory multiple myeloma, patients and their physicians have more treatment options to consider. Like the study by McKay et al, this research effort was designed to determine patient preferences using a discrete-choice experiment coupled with a best-worst scaling exercise to discover treatment priorities and unmet needs.
The discrete-choice survey included 6 features of multiple myeloma treatments with varying options. These included efficacy measured by progression-free survival (PFS; range, 6-24 months); risk of congestive heart failure (range, 0%-5%); likelihood of experiencing nerve damage, also known as peripheral neuropathy (none, mild to moderate, or severe); likelihood of experiencing low blood counts, such as low platelets and low white blood cells (range, 0%-70%); likelihood of experiencing gastrointestinal problems (none, nausea and vomiting, diarrhea, constipation); method and frequency of administration (daily and weekly pill, weekly injection, intravenous [IV] infusion 4 hours per week, IV 1 hour twice a week).
The best-worst scaling exercise included 18 questions that asked about mode and frequency of administration, other aspects of treatment convenience, mild adverse events, serious adverse events, and treatment side effects. The final survey was administered online to patients recruited from the Multiple Myeloma Research Foundation CoMMpass study.
A total of 94 patients with relapsed or refractory multiple myeloma completed the survey. The average age of these patients was 65 years, and most (59%) were male. One subgroup of patients in this group stated that toxicities, such as nerve damage, low blood counts, and gastrointestinal problems, were relatively more important than a change in PFS from 6 months to 1 year. The second subgroup considered effectiveness (specifically PFS) more important than nerve damage and the method of administration. A change in PFS from 6 months to 2 years was more than 2 times as important to these patients as the relative importance of changes in all other attributes.
| “I might really want as close to an all-oral therapy as possible if I am planning to continue working and have to travel. If I am a more elderly patient, I might want something that is going to be easy for me to take and not cause me to feel even older than I already do. If I am young, I might decide to be as aggressive as possible and go for the cure, if that is reasonable. So, I think it really depends on both the physical and the mental position of patients in terms of how they look at the different pros and cons of the various treatment protocols.”—Jack Aiello |  |
In the best-worst scaling exercise, patients stated that kidney complications and low white blood cell count are more bothersome, while taking pills once a week for 3 weeks per month or a pill taken daily were the least bothersome, or most favorable, characteristics of a treatment for multiple myeloma.
Results from this study suggest that there are subgroups of patients with different treatment preferences. Understanding how different patients value treatment attributes can help physicians and other healthcare team members improve the quality of their care for patients with multiple myeloma.
 |
“The highest-ranking treatment options are those that show good efficacy with a minimum of required encounters with the treatment center. I want my time to be my time, and going for evaluation or therapy easily takes one-half or a whole day out of my life. Oral is better than parenteral. Subcutaneous is better than an intravenous infusion. Three capsules per month are better than 21 capsules per month. Insurance coverage and co-pay requirements are essential considerations. For me, all of these factors would be the same whether it was a first, second, third, or fourth line of therapy. Personally, I have had 4 distinct lines of therapy. I felt all of my treatment choices were effective, and looked at which ones gave good QoL. Also, multiple myeloma drugs are expensive. Each drug, an immunomodulatory drug or a proteasome inhibitor, is $10,000 apiece. Throw in monoclonal antibodies, $10,000 to $15,000 or more, so it can be $25,000 a month for every course of treatment. It is just amazing that costs are so high. So, we need to rely on the companies to give financial assistance. The financial assistance can come in the way of foundations that get their funding from drug companies. And it comes, of course, from insurance coverage for private pay patients. It is a huge problem—financial toxicity is a real worry for most patients with cancer, not just myeloma.”—James Omel, MD |
Source
- Auclair D, Mansfield C, Chari A, et al. Patient treatment preferences for relapsed/refractory multiple myeloma: are patients willing to trade off efficacy for tolerability? Presented at the 2018 ASH Annual Meeting; December 3, 2018; San Diego, CA. Abstract 614.
See Appendix for a list of resources to help patients with multiple myeloma find financial help from pharmaceutical companies or financial assistance organizations.
JCARH125, Anti-BCMA CAR T-Cell Therapy for Relapsed/Refractory Multiple Myeloma: Initial Proof of Concept Results from a Phase 1/2 Multicenter Study (EVOLVE)
JCARH125 is an investigational chimeric antigen receptor (CAR) T-cell therapy that is in early-stage clinical trials as a treatment for patients with relapsed or refractory multiple myeloma. With CAR T-cell therapy, the patient’s own immune cells (T lymphocytes) are altered to be able to recognize cancer cells and more effectively target and destroy them. JCARH125 targets B-cell maturation antigen (BCMA), a protein found on the surface of malignant plasma cells, which has become an attractive therapeutic target for various treatments in development for patients with multiple myeloma. JCARH125 has been engineered to reduce side effects and improve the chance of durable responses.
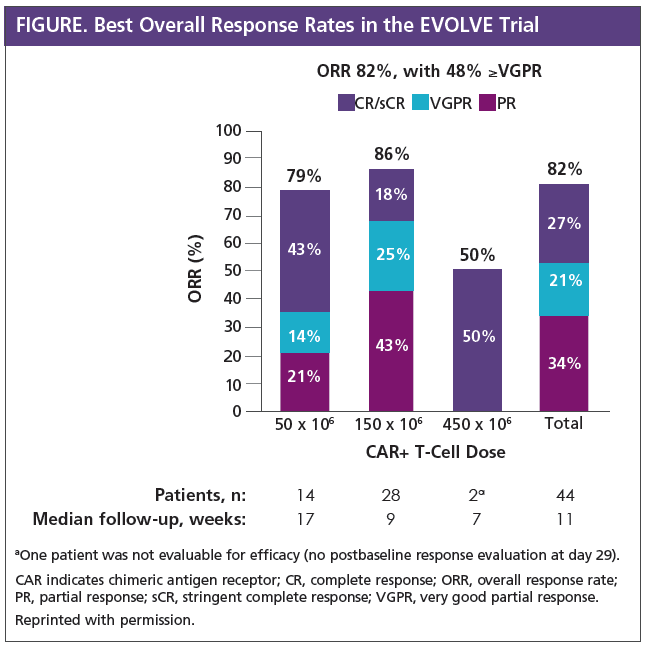
At ASH 2018, researchers presented results of an ongoing clinical trial of JCARH125 called EVOLVE. This multicenter phase 1/2 study is enrolling patients with relapsed or refractory multiple myeloma who have received 3 or more prior treatment regimens, including autologous stem-cell transplant (ASCT), a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 monoclonal antibody. In this trial, patients receive chemotherapy (fludarabine and cyclophosphamide) 2 to 7 days before a single infusion of JCARH125.
At the time of data analysis, 44 patients with relapsed or refractory multiple myeloma had received JCARH125. The median age of these patients was 62 years (range, 36-79 years), with a median time from diagnosis of 6 years (range, 2-17 years). Patients had received a median of 6 prior regimens (range, 2-17 regimens). Of these patients, 95% had undergone at least 1 prior ASCT, and 77% had high-risk disease based on cytogenetic testing. The overall response rate associated with JCARH125 was 82% in these heavily pretreated patients. Almost half (48%) of the patients had deep responses (very good partial response or better).
Side effects associated with CAR T-cell therapy include cytokine release syndrome (CRS) and neurotoxicity (NT). In this study, CRS was observed in 4 of 44 (9%) patients after a median of 3 days (range, 1-10 days) and lasted a median of 5 days (range, 1-19 days).
| “CAR-T looks like an exciting treatment for multiple myeloma. It is not considered curative at this point, but perhaps if it is used earlier in treatment, maybe, it will be one day.”—Jack Aiello |  |
Eleven of 44 (25%) patients experienced neurologic adverse events. Median onset of NT was 3 days (range, 1-12 days), with a median duration of 6 days (range, 1-58 days).
Although durability of response and response rate in a greater number of patients remain to be determined, early experience with JCARH125 supports a good benefit-risk profile and continuation of clinical development.
Source
- Mailankody S, Htut M, Lee KP, et al. JCARH125, Anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: initial proof of concept results from a phase 1/2 multicenter study (EVOLVE). Presented at the 2018 ASH Annual Meeting; December 3, 2018; San Diego, CA. Abstract 957.
Addition of Ixazomib to Lenalidomide plus Dexamethasone Improves Clinical Benefit in Patients with Relapsed/Refractory Multiple Myeloma and Non-Canonical NF-κB Activation: Results from the TOURMALINE-MM1 Trial
Ixazomib (Ninlaro) is an orally administered proteasome inhibitor (PI) currently approved by the US Food and Drug Administration in combination with lenalidomide (Revlimid) and dexamethasone for the treatment of patients with multiple myeloma who have received at least 1 prior therapy. The approval of ixazomib was based on results from the TOURMALINE-MM1 clinical trial. In this large, phase 3 trial, ixazomib (I) was shown to extend progression-free survival (PFS) when given in combination with lenalidomide and dexamethasone (Rd) in patients with relapsed or refractory myeloma who had received 2 or more prior therapies, including patients with high-risk cytogenetic abnormalities.
 |
“It is very early on with respect to using gene mutations to treat multiple myeloma, in part because the myeloma cell is very complex and there can be multiple gene mutations, in part because there is no single gene mutation in the disease that represents a large percentage of patients, and because there are a limited number of drugs that work on certain gene mutations.”—Jack Aiello |
In addition to evaluating the efficacy and safety of the ixazomib/lenalidomide/dexamethasone (IRd) combination regimen, researchers in the TOURMALINE-MM1 study wanted to find out if there are relationships between the efficacy of IRd and specific features of the patient’s disease, such as tumor gene expression patterns and mutational status. The idea of matching particular patients to particular treatments based on the genetic features of their multiple myeloma is key to personalized medicine. Ajeeta B. Dash, PhD, who presented results from this study at ASH 2018, explained that physicians strive to tailor treatment to each patient’s specific cancer whenever possible.
One method by which cancer cells grow and “talk” to each other is called the NF-κB pathway. This pathway can be canonical or non-canonical. Almost 1 in 5 (17%) multiple myeloma tumors have mutations in genes that specifically control the non-canonical NF-κB pathway. When these gene mutations are present, tumor cells may be able to resist standard treatments.
Previous trials have shown that bortezomib (Velcade) can be effective in patients whose tumors are activated or stimulated by the non-canonical NF-κB pathway. In the TOURMALINE-MM1 trial, researchers wanted to determine whether use of a different PI, ixazomib, together with Rd, is effective in patients whose tumors were activated by the non-canonical NF-κB pathway. They examined PFS results to learn whether the IRd combination is more effective than use of Rd alone for patients in this subgroup.
The researchers found that, in patients with mutations specific to the NF-κB pathway, PFS was longer in those who received IRd compared with those who received Rd alone. By showing this benefit in many trial participants, they were able to confirm that using a PI is effective in patients with molecularly defined multiple myeloma.
| “In theory, biomarkers to select treatment is a good idea, and we will get there soon, but we are not there yet in multiple myeloma. The majority of patients are going to get a regimen of lenalidomide/bortezomib/dexamethasone as induction therapy. Patients who have high-risk cytogenetics—17p deletions, 14;16 translocations, 4;14 translocations— they probably should have a PI not only in their induction treatment but also as consolidation and even maintenance. I do not think we are to the point of saying a particular biomarker equates to a specific multiple myeloma presentation.”—James Omel, MD |  |
Source
- Dash AB, Zhang J, Shen L, et al. Addition of ixazomib to an Rd backbone improves clinical benefit in relapsed/refractory multiple myeloma (RRMM) patients (Pts) with non-canonical NF-KB activation—results from the Tourmaline-MM1 study. Presented at the 2018 ASH Annual Meeting; December 2, 2018; San Diego, CA. Abstract 473.
Efficacy of Ixazomib/Lenalidomide/Dexamethasone in Routine Clinical Practice Similar to Efficacy in the TOURMALINE-MM1 Trial: Analysis from the INSIGHT MM Observational Study and the Czech Registry of Monoclonal Gammopathies
Starting in 2015, the proteasome inhibitor ixazomib (Ninlaro) given as a once-weekly pill was approved for use in combination with lenalidomide (Revlimid) and dexamethasone in more than 50 countries around the globe, including the United States and European Union, for the treatment of patients with relapsed or refractory multiple myeloma who had received at least 1 prior therapy. Physicians who treat cancer and researchers who perform clinical trials of novel treatments know that measures of outcomes and tolerability are often different between routine clinical practice and clinical trials. There are very few research efforts directly comparing the efficacy of new drugs based on clinical trial findings compared with their efficacy in routine clinical practice.
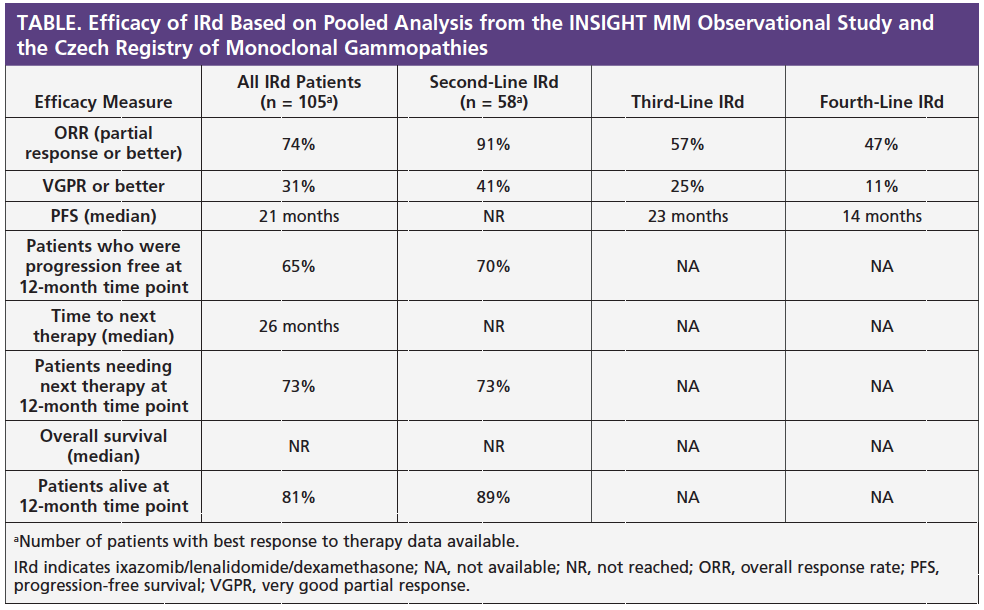
To evaluate the efficacy of the combination of ixazomib/lenalidomide/dexamethasone (IRd) in real-world patients with relapsed and refractory multiple myeloma, researchers analyzed patient-level data from the ongoing INSIGHT MM study using data from the Czech Registry of Monoclonal Gammopathies (RMG). INSIGHT MM, the largest global, prospective, observational study of its kind to date, is enrolling approximately 4200 adults with newly diagnosed or relapsed/refractory multiple myeloma from around the world: Europe, United States, Asia, and Latin America. The Czech RMG, which the Czech Myeloma Group initiated in 2007, includes clinical data for more than 6000 patients with multiple myeloma who enrolled at 1 of 19 Czech and 4 Slovak cancer centers.
After data were collected from 9 countries, 163 patients with relapsed or refractory multiple myeloma who received IRd were included in the analysis (50 from the INSIGHT MM database and 113 from the Czech RMG). Of these patients, 90% were from Europe, 10% were from the United States, and 1% were from Taiwan. Patients were aged 67 years (median; range, 39-84 years), and 53% of patients were men. At the time of initial diagnosis with multiple myeloma, 38% of patients had International Staging System (ISS) stage I disease, 36% had ISS stage II disease, and 26% had ISS stage III disease.
Most (61%) patients in this database had received a previous stem-cell transplant. Prior therapy medications included bortezomib (Velcade) in 89% of patients, thalidomide (Thalomid) in 42%, lenalidomide (Revlimid) in 21%, carfilzomib (Kyprolis) in 11%, daratumumab (Darzalex) in 3%, and pomalidomide (Pomalyst) in 2%.
 |
“When patients hear that real people use this therapy and these are the results, they feel reassured that real people are actually using it, and this is their experience. This definitely needs to be considered as extremely relevant.”—Barbara Kavanagh, MSW, LCSW |
The median time between diagnoses of multiple myeloma and starting IRd treatment was 43 months. Overall, 50% of patients received IRd as second-line therapy, 30% as third-line therapy, and 20% as fourth-line therapy. The most common reason for starting IRd therapy was relapse or progression after prior treatment (90%), including bone lesions and anemia. Patients received the IRd combination for a median of 14 months.
At the time of data analysis, results on best response to therapy were available for 105 of the 163 patients. Among all patients, the overall response rate (ORR; partial response or better) was 74%. Almost one-third (31%) of patients had a very good partial response (VGPR) or better. The ORR with IRd when given as second-, third-, or fourth-line therapy was 91%, 57%, and 47%, respectively. The frequency of VGPR or better was also higher for patients who received IRd in earlier lines of therapy.
| “I feel that IRd is a good option for treatment, and the database results would bolster my belief and acceptance of this triplet therapy. I absolutely do accept and consider real-world data. Real-world patients do not always have great renal status or meet age requirements. Their livers, hearts, and lungs may not be perfect, and they may not have great ECOG performance status, but they still need multiple myeloma therapy. Observational trials such as INSIGHT tell us what happens to these important people, who may be excluded from clinical trials because they do not meet inclusion criteria.”—James Omel, MD |  |
Results showed that 23% of the patients who relapsed after treatment with IRd received subsequent therapies. These included bortezomib (24%), pomalidomide (24%), thalidomide (16%), daratumumab (16%), carfilzomib (14%), or lenalidomide (8%).
Reductions in the dose of ixazomib were required in 15% of patients, while reductions in the lenalidomide dose were required in 30% of patients.
These findings indicate that the efficacy of IRd in routine clinical practice is similar to the efficacy of IRd as reported in the regimen’s pivotal trial, TOURMALINE-MM1. In this trial, the ORR associated with IRd was 78% and median progression-free survival was 21 months.
Researchers also concluded that IRd is well-tolerated in patients with relapsed and refractory multiple myeloma who receive treatment in clinical practice.
Source
- Hajek R, Terpos E, Lee HC, et al. Ixazomib plus lenalidomide-dexamethasone (IRd) in relapsed/refractory multiple myeloma (MM) patients (pts)—effectiveness in routine clinical practice is similar to the efficacy in the phase 3 Tourmaline-MM1 trial: a pooled analysis from the Insight MM observational study and the Czech Registry of Monoclonal Gammopathies (RMG). Presented at the 2018 ASH Annual Meeting; December 1, 2018; San Diego, CA. Abstract 1971.
Maintenance Therapy
Treatment Choices and Outcomes for Patients with Multiple Myeloma After Relapse on Lenalidomide Maintenance Therapy: Results from the Connect® Multiple Myeloma Registry
There are many effective treatment choices for patients with multiple myeloma whose disease progresses while receiving lenalidomide (Revlimid) maintenance therapy. Today, most physicians talk with their patients about the pros and cons of each of these treatment options, and they choose the next treatment together. There is no established sequence in which treatments for relapsed multiple myeloma should be given, and patterns of treatment vary.
To better understand treatment patterns in real-world patients with multiple myeloma, Connect® MM–The Multiple Myeloma Disease Registry was established. Connect MM is an observational cohort study that researchers use to learn about diagnostic and treatment patterns, clinical outcomes, and quality of life in patients with multiple myeloma. Using data from this registry, researchers can describe patterns of relapse after lenalidomide maintenance, specific treatment choices after relapse, and efficacy outcomes associated with those treatments.
The Connect MM study enrolled 3011 transplant-eligible and transplant-ineligible adults who were newly diagnosed with multiple myeloma from 250 different cancer care centers. Patients were treated at physicians’ discretion and then followed up to determine if and how they responded to treatment in the relapsed setting.
 |
“For today’s multiple myeloma patient, it is important to get educated about the disease. Understand what questions you should be asking your doctor. Understand what markers are being used to track responses to treatment. Share with your doctor any kind of side effects you are experiencing. Very importantly, if at all possible, have a multiple myeloma expert be part of your team. You could go to essentially any major city and get a second opinion from a doctor who then becomes part of your medical team that can advise your community oncologist as to what may be the next best treatment for you.”—Jack Aiello |
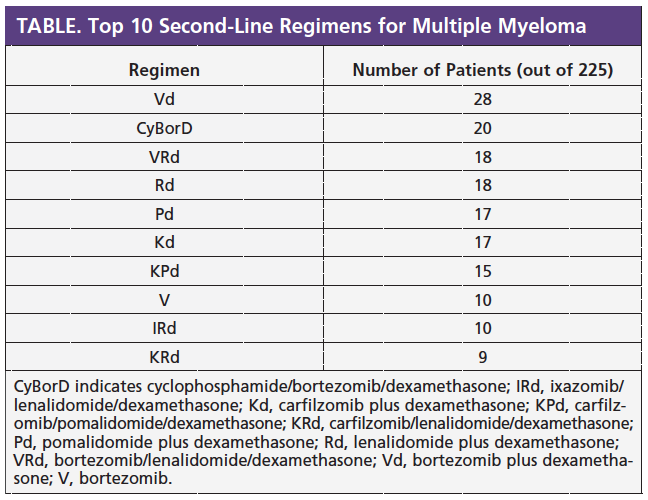
As of January 2018, Connect MM included more than 1100 patients who started second-line therapy, of whom 236 had received lenalidomide maintenance. Among these 236 patients, 52% (n = 123) experienced symptoms, such as high calcium levels, kidney failure, anemia, and bone disease, when they relapsed, whereas the other 47% (n = 112) were asymptomatic. The primary research question was whether progression-free survival (PFS) from the start of second-line therapy was different between patients who were symptomatic and those who were asymptomatic. Investigators also reviewed safety and adverse events from the start of second-line treatment.
The Table shows the top 10 second-line regimens these patients received. Regimens included both triplet+ regimens (3 or more drugs combined) and doublet regimens (1 drug or 2 drugs combined). Specifically, approximately 50% of patients switched to proteasome inhibitor (PI) regimens without immunomodulatory drugs (IMiDs), 25% continued with an IMiD plus a PI, and 25% continued with an IMiD.
Using Connect MM data, researchers learned that patients who received triplet+ regimens in the second-line setting had longer median PFS compared with patients who received doublet regimens as second-line therapy. PFS was longest in patients who received an IMiD plus a PI compared with patients who received a PI without an IMiD.
Rates of serious adverse events were similar for patients receiving various types of treatments in the second-line setting: 43% of patients receiving an IMiD plus a PI had serious adverse events, compared with 35% of those taking an IMiD without a PI, and 36% of those taking a PI without an IMiD.
This is the first description of relapse patterns, second-line treatment choice, and survival outcomes after progressive disease on lenalidomide maintenance therapy in community-based patients. Nearly equal numbers of patients were either symptomatic or asymptomatic at the time of relapse on lenalidomide maintenance therapy.
Source
- Jagannath S, Narang M, Ailawadhi S, et al. Treatment choices and outcomes for patients with multiple myeloma after relapse on lenalidomide maintenance therapy: results from the Connect® Multiple Myeloma Registry. Presented at the 2018 ASH Annual Meeting; December 2, 2018; San Diego, CA. Abstract 3232.
Maintenance Therapy with Ixazomib Significantly Prolongs Progression-Free Survival Following Autologous Stem-Cell Transplantation in Patients with Newly Diagnosed Multiple Myeloma: Results from the Phase 3 TOURMALINE-MM3 Trial
In patients with multiple myeloma, the use of drug therapy as maintenance has been shown to prolong the length of time the disease is controlled. Specifically, use of maintenance therapy may affect overall survival, particularly when it is used after an autologous stem-cell transplant (ASCT).
| “The important part [of TOURMALINE-MM3] is that about 30% of patients on Revlimid cannot take the drug, so clearly now those individuals have another option with ixazomib as maintenance. It is a simple oral maintenance taken once a week.”—Jack Aiello |  |
For several years, lenalidomide (Revlimid)—an immunomodulatory drug (IMiD) that is taken orally—has been the only medication approved by the US Food and Drug Administration (FDA) specifically for use as maintenance therapy in multiple myeloma. Although lenalidomide is clearly effective as maintenance therapy, its use can increase the chance of side effects as well as the risk for developing a second cancer.
Proteasome inhibitors (PIs) are another class of drugs used as a backbone of multiple myeloma treatment. As of December 2018, 3 PIs have been approved for use in multiple myeloma in the United States: bortezomib (Velcade), which is given by subcutaneous injection or intravenous infusion; carfilzomib (Kyprolis), which is given by intravenous infusion; and ixazomib (Ninlaro), which is given orally.
Bortezomib-based maintenance therapy has shown some evidence of activity in patients who have undergone ASCT, but this benefit was not demonstrated when the treatment was compared with placebo in a phase 3 trial. Bortezomib maintenance is also limited by concerns regarding side effects and the need for regular injections or infusions.
Ixazomib is an orally administered PI that is currently approved by the FDA in combination with lenalidomide and dexamethasone for patients with multiple myeloma who have received at least 1 prior therapy. Because its oral administration is potentially more convenient for patients, researchers were interested in learning whether ixazomib could be used as maintenance therapy.
To assess the value of ixazomib maintenance therapy in patients with multiple myeloma, investigators initiated a phase 3, double-blind, placebo-controlled, multicenter clinical trial called TOURMALINE-MM3. This trial compared weekly ixazomib maintenance with placebo in patients with newly diagnosed multiple myeloma who had at least a partial response to their induction therapy with a PI (bortezomib) and/or an IMiD (lenalidomide) followed by single ASCT.
Patients received ixazomib or placebo on days 1, 8, and 15 of 28-day cycles for up to 2 years or until progressive disease or unacceptable toxicity. The ixazomib dose was 3 mg during the first 4 cycles, and then was increased to 4 mg from cycle 5 onward if it was tolerated well during cycles 1 to 4.
The primary measure of the efficacy of ixazomib maintenance was progression-free survival (PFS), which was assessed by an independent review committee of physicians who were blinded to treatment assignment. A key secondary measure of the efficacy of ixazomib maintenance was overall survival. At ASH 2018, researchers reported data from the final assessment of PFS.
A total of 656 patients enrolled in the TOURMALINE-MM3 trial; 395 received ixazomib maintenance and 261 received placebo. Median age in both groups was 57 years (range, 24-73 years), and more than half (59%) had received a PI without an IMiD during their induction therapy. Most (79%) of these patients had achieved a complete response or very good partial response following induction along with ASCT. A minority (18%) of patients in this trial had high-risk cytogenetics (del[17p], t[4;14], or t[14;16]).
 |
“Most patients will say, ‘If it is going to prolong my life, I am willing to deal with that.’ So, I think, in balance, once the patient and family know both the risks and the benefits of a new therapy, most of them would say, ‘I am going to give this a try.’”—Barbara Kavanagh, MSW, LCSW |
After following these patients for an average of 31 months, researchers in this trial observed a 28% reduction in the risk for progression or death for those who received ixazomib compared with those who received placebo. Stated differently, there was a 39% improvement in PFS with ixazomib compared with placebo. The median time over which patients were free from progressive disease improved by approximately 6 months for those receiving ixazomib (27 months) compared with those receiving placebo (21 months). This difference in median PFS was statistically significant.
Patients who underwent ASCT after their induction regimen and who received ixazomib had longer PFS compared with those who received placebo maintenance. The median PFS for patients who underwent ASCT and then received ixazomib maintenance was 31 months compared with 25 months for placebo. Ixazomib maintenance also led to a higher percentage of patients with no evidence of minimal residual disease compared with placebo (12% vs 7%).
The benefit in PFS was seen across all subtypes of multiple myeloma, including patients with high-risk disease, those who received a PI as a part of induction, and those who did not receive a PI as a part of induction.
In the TOURMALINE-MM3 trial, 7% of patients in the ixazomib group and 5% of patients in the placebo group discontinued maintenance therapy because of adverse events. Approximately one-quarter (27%) of patients receiving ixazomib experienced 1 or more severe adverse events compared with 20% of patients receiving placebo. Frequently observed severe adverse events associated with ixazomib versus placebo were infections (15% vs 8%), such as pneumonia (6% vs 4%), gastrointestinal disorders (6% vs 1%), low white blood cell count (5% vs 3%), and low platelet count (5% vs <1%). Peripheral neuropathy (any severity level) was reported by 19% of patients in the ixazomib group and 15% of those in the placebo group. The rate of second primary cancers was low (3%) in both study groups. Quality-of-life (QoL) survey scores were similar between patients receiving ixazomib and those receiving placebo, suggesting that maintenance therapy with ixazomib did not negatively affect QoL.
In summary, the TOURMALINE-MM3 trial demonstrated a 28% reduction in risk for progression or death, corresponding to a 39% improvement in PFS for maintenance therapy with ixazomib. Over time, researchers noted deepening of responses and more conversions to minimal residual disease negativity, as well as a good safety profile, including low rates of peripheral neuropathy. They concluded that ixazomib is valuable as a future option for maintenance therapy in patients with multiple myeloma, particularly those who have undergone ASCT.
| “It is very telling for me that QoL scores for ixazomib were similar to placebo—the drug is well-tolerated. In general, the TOURMALINE-MM3 trial did what it was expected to do…it met its primary objective and showed the value of ixazomib in maintenance therapy. For the 30% of patients who cannot tolerate lenalidomide maintenance and have to stop therapy, ixazomib is now proven to be a good alternative.”—James Omel, MD |  |
Source
- Dimopoulos MA, Gay F, Schjesvold FH, et al. Maintenance therapy with the oral proteasome inhibitor (PI) ixazomib significantly prolongs progression-free survival (PFS) following autologous stem cell transplantation (ASCT) in patients with newly diagnosed multiple myeloma (NDMM): phase 3 Tourmaline-MM3 trial. Presented at the 2018 ASH Annual Meeting; December 2, 2018; San Diego, CA. Abstract 301.
Quality of Life
The Impact of Lenalidomide/Bortezomib/Dexamethasone Treatment on Health-Related Quality of Life in Transplant-Eligible Patients with Newly Diagnosed Multiple Myeloma: Results from the IFM/DFCI 2009 Trial
Multiple myeloma can be a debilitating disease, and many patients are burdened by pain, fatigue, and other symptoms, such that their health-related quality of life (HRQoL) is lower than people of the same age who do not have a diagnosis of cancer.
A presentation at ASH 2018 focused on change in HRQoL in patients with multiple myeloma who had participated in a large, randomized clinical trial conducted in 2009 by the Intergroupe Francophone du Myélome, a French study group, and the Dana-Farber Cancer Institute in Boston, MA (IFM/DFCI). The primary objective of the IFM/DFCI trial was to assess whether use of a triplet (3-drug) regimen—specifically lenalidomide (Revlimid)/bortezomib (Velcade)/dexamethasone (RVD)—was effective as induction and consolidation therapy in patients newly diagnosed with multiple myeloma, including those who had undergone stem-cell transplantation (SCT). All patients in this trial received lenalidomide maintenance for 12 months (Attal M et al. Blood. 2015;126:391).
In this trial, the combination of RVD followed by SCT and lenalidomide maintenance was found to significantly prolong progression-free survival in patients who were newly diagnosed with multiple myeloma. However, the use of RVD did not extend overall survival.
The researchers measured HRQoL using standardized surveys, including the European Organisation for Research and Treatment of Cancer Quality of Life Core 30 questionnaire and the multiple myeloma module (QLQ-MY20). These surveys ask patients to rate their overall quality of life (QoL), ability to function physically, ability to function in their roles, fatigue, pain, side effects of treatment, and disease symptoms.
Patients with multiple myeloma were surveyed on 9 occasions (including at the time they enrolled in the IFM/DFCI study, during induction treatment with RVD, during consolidation therapy, while on maintenance with lenalidomide, at the end of treatment, and during follow-up visits). Patients’ survey responses regarding HRQoL were compared with average scores from the general population to help interpret the study findings.
At the end of induction therapy with RVD, patients in the IFM/DFCI trial reported improvements in global QoL, physical functioning, role functioning, and pain compared with baseline (time of trial enrollment). Their reported levels of fatigue did not change. In the QLQ-MY20 questionnaire, disease symptoms improved but side effects of treatment did not.
Among patients who received RVD followed by SCT, QoL worsened in the short-term following SCT, but scores gradually improved over time. However, all key QoL domains improved over time at the end of induction therapy and continued to improve during consolidation and maintenance treatment. These improvements were maintained throughout the posttreatment follow-up period. At the time of patients’ second follow-up visit, QoL scores were at a level that was mostly similar to people in the general population.
In summary, this research regarding HRQoL showed that patients with multiple myeloma who enrolled in the IFM/DFCI 2009 trial and who received RVD benefited both clinically—based on evidence of disease control—and in terms of HRQoL.
Source
- Roussel M, Hebraud B, Hulin C, et al. The impact of lenalidomide, bortezomib, and dexamethasone treatment on health-related quality of life in transplant-eligible patients with newly-diagnosed multiple myeloma: results from the IFM/DFCI 2009 trial. Presented at the 2018 ASH Annual Meeting; December 3, 2018; San Diego, CA. Abstract 716.
Health-Related Quality of Life in Patients with Relapsed/Refractory Multiple Myeloma Who Received Pomalidomide/Bortezomib/Low-Dose Dexamethasone versus Bortezomib plus Low-Dose Dexamethasone: Results from the OPTIMISMM Trial
Patients who are diagnosed with multiple myeloma can experience symptoms of the disease and treatment-related side effects that negatively affect their health-related quality of life (HRQoL). Symptoms of multiple myeloma, such as bone pain, infection, and fatigue, can be troublesome, particularly after a patient has undergone several lines or types of therapy.
At ASH 2018, researchers presented an analysis of patients’ quality of life (QoL) using data from the phase 3 OPTIMISMM trial. This was a large trial (N = 559) showing that patients with multiple myeloma who received a triplet (3-drug) combination regimen of pomalidomide (Pomalyst)/bortezomib (Velcade)/low-dose dexamethasone (PVd) had significantly longer progression-free survival (PFS) and higher overall response rate compared with patients with multiple myeloma who received bortezomib plus low-dose dexamethasone (Vd). All patients in this trial had received previous treatment with lenalidomide (Revlimid).
 |
“Because of chemotherapy and radiation therapy, I have lower QoL than when I was originally diagnosed and treated. Would I have exchanged that for the shorter overall survival? No. I am still solid from the neck up and have taken those 24 years and looked back at it as the fact that I am very fortunate to be around.”—Jack Aiello |
| “My husband (the patient who developed peripheral neuropathy because of therapy) would look at you and say, ‘There are side effects to every drug, and you have to adapt.’ The family has to adjust. It is not only QoL. Very often, patients cannot continue their normal work, or their activities, but when you speak to that person, he or she would say, ‘I am glad I am still alive!’”—Barbara Kavanagh, MSW, LCSW |  |
Knowing that HRQoL in relapsed/refractory multiple myeloma is an important consideration, researchers looked further into the data collected to evaluate the effect of PVd and Vd on HRQoL. To understand whether these treatments affected QoL, and if so, how, investigators used a specific survey called the European Organisation for Research and Treatment of Cancer Quality of Life Core 30 questionnaire. A total of 449 patients (240 patients in the PVd group and 209 in the Vd group) completed this survey at multiple time points in the study, including day 1 of each 21-day treatment cycle before treatment administration and at the end of treatment.
Demographic characteristics of the patients, such as age, time since diagnosis, and number of previous treatments, were similar between those who received PVd and those who received Vd. Global QoL scores were also similar between groups at the start of the study.
When comparing the 2 groups’ QoL scores over time, researchers found that there were no clinically meaningful differences between patients who received PVd and those who received Vd. There was also no difference in the proportion of patients who experienced clinically meaningful worsening in their global QoL between the treatment groups.
The results from this analysis of the OPTIMISMM trial showed that the triplet regimen of PVd did not worsen HRQoL in patients with relapsed or refractory multiple myeloma.
 |
“QoL is a very important outcome measure for patients with multiple myeloma. PFS and overall survival (OS) are important too, of course, but if 2 arms of a clinical trial have generally similar PFS and OS results, I would definitely choose the better QoL treatment. However, I would definitely choose a therapy that improved my clinical outcome if it had little or no impact on QoL. I would NOT choose that therapy if it worsened my QoL, even if it modestly improved survival. I do not want to extend my life if the price to do it is feeling miserable for a few more months. On the other hand, if the survival difference was quite large, my decision might be different.”—James Omel, MD |
Source
- Weisel K, Dimopoulos MA, Moreau P, et al. Health-related quality of life among patients with relapsed or refractory multiple myeloma who received pomalidomide, bortezomib, and low-dose dexamethasone versus bortezomib and low-dose dexamethasone—results from the phase 3 OPTIMISMM study. Presented at the 2018 ASH Annual Meeting; December 1, 2018; San Diego, CA. Abstract 1960.
Patient-Reported Outcome–Driven Case Management System for Hematology: A Prospective Study
The time available for physicians and other healthcare providers to talk with their patients with cancer, including those with multiple myeloma, and to gather all the information needed to make good decisions is restricted in a typical office visit. The ability of the care team to meet patients’ needs within these time constraints is challenging, such that both patients and clinicians are frustrated and worried about less-than-optimal outcomes.
To address this issue, researchers developed and tested an electronic point-of-care case management system for patients with cancer. The goal of this system was to obtain patient feedback about their quality of life (QoL) and give physicians key information about QoL issues that need to be addressed.
| “I would rather have an old-fashioned face-to-face talk with my physician instead of writing my responses on an electronic data capture device. I would also like my physician to look at me instead of his computer screen. Unfortunately, what I would like has been affected by the realities of delivering medical care at this stage. I do not like the electronics, but they are a part of medicine, and we all must adjust.”—James Omel, MD |  |
To determine whether this QoL reporting system helped with patient satisfaction, researchers tested it in a prospective randomized study in comparison with usual care. The primary goal of the study was to learn whether patients who used the point-of-care system would enhance their QoL over time.
The 233 patients who participated in this research effort were adults diagnosed with multiple myeloma (n = 171) or light amyloidosis (n = 62) who were seen at the Mayo Clinic. The median age of patients who participated in the research was 65 years (range, 31-87 years). Median time from diagnosis to study consent was 37 months, and most (59%) were male.
Using the QoL reporting system, patients selected their “single biggest concern” from a list of categories and then received a printed list of resources that might help the individual patient based on the selected concern. Clinicians also received the patient-reported QoL results to review with patients. The average time that was required for patients to complete the survey was 6 minutes.
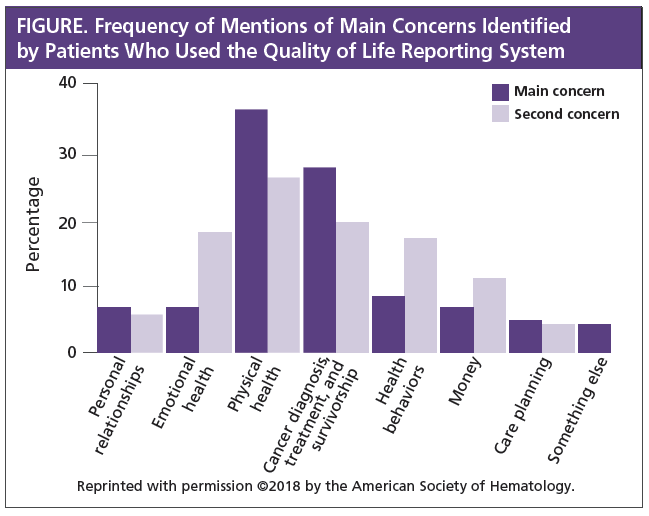
The most common concerns that these patients chose in the QoL reporting system were “physical health” (37%) and “cancer diagnosis” (27%; see Figure). This pattern remained, even when patients were given the option to pick a second major concern. No other concern was listed by more than 8% of patients. Using this system, patients also indicated a strong desire for more information about their therapy, clinical trials, their likely outcome (prognosis), and drug-related side effects.
After the research was complete, patients stated that they found the QoL reporting system worth their time (75%) and that they would choose to use it again (87%) and recommend the system to others (82%). Among clinicians, 82% thought the patient-reported QoL tool was neutral or positive in terms of its effect on their practice. Most (75%) believed that they might see improvements in patient well-being.
Upon completing the research, investigators learned that the patient-reported QoL system was well-received by patients and clinicians. However, it did not improve patients’ QoL relative to usual care. Researchers concluded that further investigation is needed to understand both why QoL did not improve and the key factors that limit communication (that is, time or how the information is delivered [verbal or written]).
Source
- Warsame R, Fruth B, Croghan K, et al. Patient-reported outcome driven case management system for hematology – a prospective study. Presented at the 2018 ASH Annual Meeting; December 3, 2018; San Diego, CA. Abstract 719.
Final Thoughts on Trial Results Presented at ASH 2018
 |
“One takeaway from ASH 2018 is that CAR-T looks like an exciting treatment for multiple myeloma. It is not considered curative at this point, but perhaps if it is used earlier in treatment, it will be one day. Another takeaway was the different studies that were offered on populations of multiple myeloma patients that really need help—patients who are older or frail or unfit, and patients who are high risk. The other area that I thought was quite interesting is the fact that at least 10 different types of induction therapy are being evaluated. These days, you almost have a standard induction therapy for most patients with multiple myeloma…RVD. As standard as RVD is, there are other options out there. Will we move to a 4-drug therapy that adds daratumumab? Is carfilzomib a better induction PI than bortezomib? What about IRd as an all-oral induction therapy? These are 3 highlights that I came away with from the presentations.”—Jack Aiello |
| “Patients are interested in what the best is and what is new. It gives them hope, and not only that, it gives them information with which they can go back to their own doctor.”—Barbara Kavanagh, MSW, LCSW |  |
 |
“The most meaningful developments in multiple myeloma therapy are the remarkable advances we are seeing in the area of immunotherapies. We now have 2 good monoclonal antibodies with a third one (isatuximab) in development. B-cell maturation antigen is proving to be a good antigen and many companies are pursuing CAR-T therapy for this target. CAR-T therapy with T-cell modification is advancing. In addition, bi-specific antibody therapy, in which an antibody bound to a drug or to cytotoxic T-cells also binds to surface antigens on malignant plasma cells, will continue to improve with time, and costs should come down. The only way to totally eliminate all multiple myeloma cells is to harness the unequaled power of the immune system because only it can keep up with myeloma cells that are constantly changing.”—James Omel, MD |
Appendix: Resources for Multiple Myeloma Patients and Their Caregivers
Pharmaceutical Reimbursement Assistance Programs
|
Amgen ASSIST 360™ Bristol-Myers Squibb Access Support® Celgene Patient Support® Janssen CarePath |
Novartis Oncology Patient Support Takeda Oncology Co-Pay Assistance Program Takeda VELCADE Reimbursement Assistance Program (VRAP) |
Other Resources
|
Benefits.gov CancerCare Financial Assistance Program Centers for Medicare & Medicaid Services HealthWell Foundation Multiple Myeloma Medicare Access (Medicare patients only) Leukemia & Lymphoma Society (LLS) Financial Support NeedyMeds |
Novartis Oncology Patient Support Takeda Oncology Co-Pay Assistance Program Takeda VELCADE Reimbursement Assistance Program (VRAP) |
Other Resources
|
Academy of Oncology Nurse & Patient Navigators American Association for Cancer Research CURE®: Cancer Updates, Research & Education |
International Myeloma Foundation www.myeloma.org/sites/default/files/images/pages/acronyms.pdf National Cancer Institute |