October 2018 Vol 4 No 5

Knowing if the patient’s chronic lymphocytic leukemia (CLL) has genetic abnormalities through genetic testing can help doctors select the best treatment. Read More ›
In July 2018, the FDA approved Tibsovo (ivo-sidenib; from Agios Pharmaceuticals), the first IDH1 inhibitor, for the treatment of adults with relapsed (returning) or refractory (not responding to treatment) acute myeloid leukemia (AML) and a susceptible IDH1 genetic mutation. Read More ›
Previously approved for thyroid cancer and kidney cancer, the FDA has recently approved Lenvima as the first treatment for patients with liver cancer. Read More ›
The use of Opdivo with Yervoy is the first immunotherapy combination therapy approved by the FDA for patients with kidney cancer. Read More ›
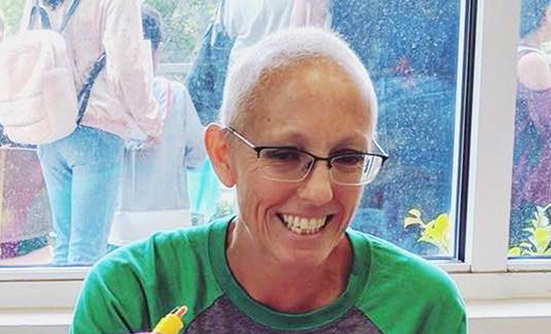
Breast cancer survivor Ginger Modiri cautions those in remission to avoid becoming complacent and to keep up with their follow-up screenings. Read More ›
Dr. Citrin explains the role of 5 experts who are essential for meeting the physical, emotional, and therapy needs of patients with breast cancer. Read More ›
Sharon Chappell expresses her experience with breast cancer through typography, art, and poetry. Read More ›