TLG1784-3
Non–small-cell lung cancer, or NSCLC, is the most common form of lung cancer, accounting for about 80% to 85% of all lung cancer cases in the United States.1 Given the complexity of cancer, the rapid development of new treatments, and the large amount of information available, it may be challenging for patients to find materials to help them understand their disease. The goal of this 4-part series of articles is to present brief overviews of concepts that may be important to caregivers and patients with NSCLC. In this third article, we describe the importance of biomarker testing in lung cancer, and provide a list of questions for patients to ask their doctors to learn more about the disease.
What Is A Biomarker?
A biomarker is a molecule that can be measured in the blood or tissue of a patient that may be a sign (marker) of an abnormality or disease. In cancer, biomarkers refer to proteins, genes, and other molecular changes that affect how cancer cells grow, multiply, respond to other compounds in the body, and die. The biomarkers measured in patients with cancer are specific to the tumor and are often caused by genetic mutations (changes) acquired at some point of a person’s life and can cause normal tissue to become cancerous.
The acquired genetic biomarkers discussed here are distinct from biomarkers that are inherited from a parent. Although a few of the mutations that are often evaluated in patients with NSCLC do run in families, such cases are rare. The majority of biomarkers found in patients with NSCLC are acquired DNA changes that are localized to the cancer itself and therefore there is no risk that a child will inherit such a mutation from a parent.
Cancer biomarkers can differ from patient to patient and even between different tumors of the same patient, which allows doctors to understand each cancer’s unique characteristics. For instance, biomarkers may allow doctors to determine which specific genetic changes are contributing to the tumor’s growth. This information can then inform doctors about which therapies an individual patient’s cancer may best respond to.2,3
Why Is Biomarker Testing Important?
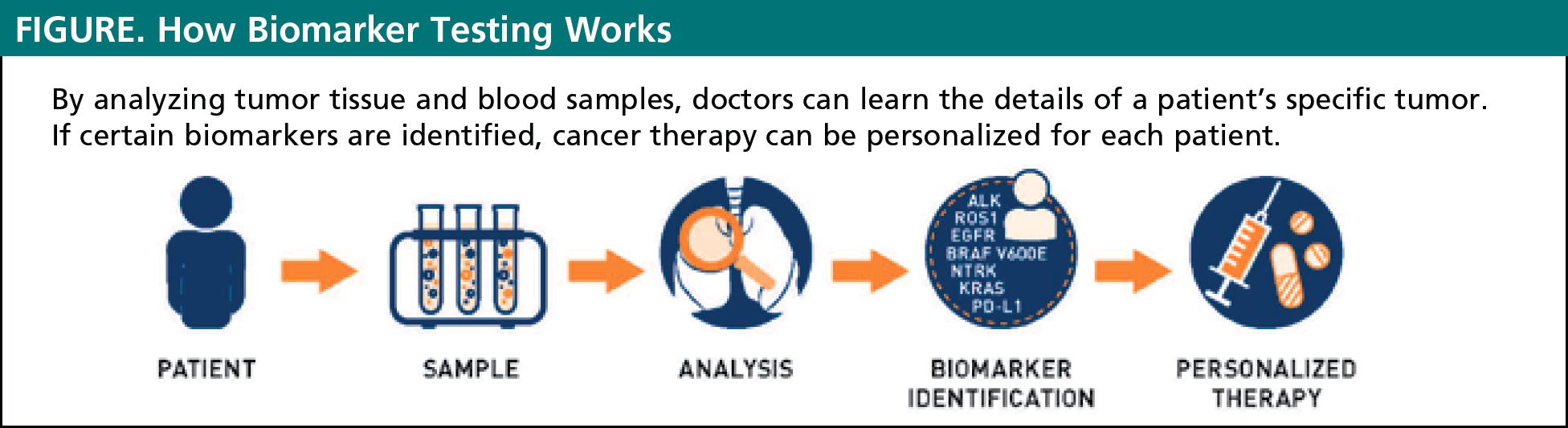
Biomarker testing is a way for doctors to gather information about a patient’s unique lung cancer. The results of these biomarker tests will help to determine if a specific cancer is likely to be improved by currently available targeted therapies or immunotherapies. Biomarker testing allows for personalized treatment plans for every unique patient (see Figure). In the past, all patients who had the same type and stage of lung cancer received the same treatment. Today, we know that there is no one-size-fits-all approach to treatment of lung cancer. Some cancers respond better to certain treatments and do not respond to other treatments. By evaluating the presence of biomarkers in a patient with cancer, doctors can often choose the treatments that are most likely to work best in an individual patient.2
Remember that not all patients may receive biomarker testing, especially those with early stages of lung cancer, whose treatment plan would not change based on their biomarkers. Other patients with later stages of lung cancer may need to repeat biomarker testing, because their cancer can change over time. For instance, in patients whose cancer relapses (returns) after treatment, doctors may need to do another biomarker test to see if the tumor has changed, which may affect the choice of treatment.
How Is Biomarker Testing Done?
Biomarker testing requires a sample of cells from the tumor that can be collected by using one of the following procedures.
- Biopsy: taking a small sample of cells from the tumor
- Surgery: collecting cells from tumors that have been removed during surgery
- Blood: looking for circulating tumor DNA in the patient’s blood.
Tissue biopsies are the “gold standard” for confirming a diagnosis of lung cancer and for performing biomarker testing, whereas liquid biopsies (which only require a blood sample) can only be used for biomarker testing, not for cancer diagnosis.4
Regardless of how the samples are collected, they will be sent to a pathologist, who is a doctor that specializes in testing cells to find disease. Biomarker test results are generally available within a few weeks (see Box).
Waiting for Test Results
When receiving a diagnosis of cancer, many patients want to initiate treatment as quickly as possible, and it is understandable that waiting a few weeks for the test results can make patients anxious.
Comprehensive biomarker panels can take time to accumulate all the results, but it is important that doctors receive the results they believe they need before making a treatment decision. In doing so, they can ensure that the right therapy is given to the right patient at the right time.
Although biomarkers can be a powerful tool in current cancer treatment, doctors may not test for any or all possible biomarkers, especially if the tumor sample is small, or if testing would not change the treatment plan.
Nevertheless, patients should consider asking their care provider about comprehensive genomic profiling or broad molecular profiling (comprehensive biomarker testing), to ensure that all their therapeutic options are being considered.
When Should Biomarker Testing Be Done?
For patients with suspected stage III or stage IV lung cancer, doctors may suggest biomarker testing if they are performing a biopsy and have enough tissue to run the tests. Based on current practice guidelines, doctors may also suggest biomarker testing for patients who have already been diagnosed with NSCLC, or in cases in which the lung cancer has relapsed (came back) after treatment.5
Regardless of where the patients are in their cancer journey, patients with lung cancer should consider discussing biomarker testing with their doctors.6
Which Biomarkers Determine The Best Treatment In Lung Cancer?
Currently, 2 types of biomarkers are used to help doctors optimize the treatment plan for a patient with lung cancer. These include:
- Mutations in genes involved in driving cell growth (called “driver mutations”). Patients with cancers who have these mutations are candidates for treatment with targeted therapy. Examples of these biomarkers include ALK, EGFR, ROS-1, NTRK, and BRAF V600E mutations.2
- Proteins on the surface of cancer cells that prevent these cells from being detected by the patient’s immune system. Cancers with these proteins are candidates for treatment with immunotherapy. The protein PD-L1 is currently the only approved biomarker that can be used to determine the use of immunotherapy in lung cancer.2
What Do The Test Results Mean?
The results of these tests will tell doctors if a patient’s lung cancer is likely to respond to certain treatments. By knowing a tumor’s biomarker status, doctors can create an individualized treatment plan for each patient’s cancer.
What Questions Should Patients Ask Their Doctor?
- What are you trying to find with the biomarker tests?
- Have I already had any biomarker tests? Which ones? If so, what are my results? What does this mean for me? How will the results affect my treatment?
- Can I have a liquid biopsy at the same time as biomarker testing? How are the tests performed?
- Are there any complications or side effects from these tests?
- How long will it take to get the test results?
- Are there any limitations of biomarker testing?
- What does it mean if the test results are negative or not clear?
- Are there any medications that target my type of lung cancer?
- Will I need these tests again? If so, why and when?
- What if I have more than 1 positive mutation?
- Which treatment is appropriate for me?
References
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clinics in Chest Medicine. 2011;32(4):605-644.
- Villalobos P, Wistuba II. Lung cancer biomarkers. Hematology/Oncology Clinics of North America. 2017;31(1):13-29.
- MyCancer. What are biomarkers? The growing importance of biomarkers in cancer. www.mycancer.com/resources/what-are-biomarkers/.
- Rolfo C, Mack PC, Scagliotti GV, et al. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC. Journal of Thoracic Oncology. 2018;13(9):1248-1268.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Non-Small Cell Lung Cancer. Version 4.2020. www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- LUNGevity. Lung cancer treatments: what you need to know about biomarker testing. November 2017. https://lungevity.org/sites/default/files/request-materials/LUNGevity-biomarker-testing-booklet-112817.pdf.
Articles in this Series
- Staging of Non–Small-Cell Lung Cancer: A Guide for Patients
- Treatment of Non–Small-Cell Lung Cancer: A Guide for Patients
- Biomarker Testing in Non–Small-Cell Lung Cancer: A Guide for Patients
- The Patient Journey in Non–Small-Cell Lung Cancer: An Overview